Intermediate filament
|
Intermediate filaments (IFs) are a family of related proteins that share common structural and sequence features. Intermediate filaments have an average diameter of 10 nanometers, which is between that of actin (microfilaments) and microtubules, although they were initially designated 'intermediate' because their average diameter was between those of narrower microtubules and wider myosin filaments[1]. Most types of intermediate filaments are cytoplasmic, but one type, the lamins, are nuclear.
Structure

The domain structure of IF molecules is conserved. Each protein has a non-alpha-helical (globular) domain at the N and C-termini which surrounds the alpha-helical rod domain. The basic building block for IFs is a parallel and in register dimer. The dimer is formed through the interaction of the rod domain to form a coiled coil. Cytoplasmic IF assemble into non-polar unit-length filaments (ULF) which then assemble into longer structures. Part of the assembly process includes a compaction step, in which ULF tighten and assume a smaller diameter. The reasons for this compaction are not well understood, and IF are routinely observed to have diameters ranging between 7 and 12nm.
The anti-parallel orientation of tetramers means that, unlike microtubules and microfilaments which have a plus end and a minus end, IFs lack polarity.
Cytoplasmic IF do not undergo treadmilling like microtubules and actin fibers, but they are dynamic. For a review see: [1].
Types
There are about 70 different genes coding for various intermediate filament proteins. However, different kinds of IFs share basic characteristics: they are all polymers that generally measure between 9-11 nm in diameter when fully assembled.
IF are subcategorized into six types based on similarities in amino acid sequence and protein structure.
Types I and II - Acidic and Basic Keratins
-
For more details on this topic, see cytokeratin.
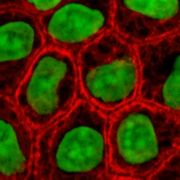
These proteins are the most diverse among IFs and constitute type I (acidic) and type II (basic) IF proteins. The many isoforms are divided in two groups:
- epithelial keratins (about 20) in epithelial cells (image to right)
- trichocytic keratins (about 13) (hair keratins) which make up hair, nails, horns and reptilian scales.
Regardless of the group, keratins are either acidic or basic. Acidic and basic keratins bind each other to form acidic-basic heterodimers and these heterodimers then associate to make a keratin filament.
Type III
There are four proteins classed as type III IF proteins which may form homo- or heteropolymeric proteins.
- Desmin IFs are structural components of the sarcomeres in muscle cells.
- GFAP (glial fibrillary acidic protein) is found in astrocytes and other glia.
- Peripherin found in peripheral neurons.
- Vimentin, the most widely distributed of all IF proteins, can be found in fibroblasts, leukocytes, and blood vessel endothelial cells. They support the cellular membranes and keep some organelles in a fixed place within the cytoplasm.
Type IV
- α-Internexin
- Neurofilaments - the type IV family of intermediate filaments that is found in high concentrations along the axons of vertebrate neurons.
- Nestin
- Synemin
- Syncoilin
Type V - Nuclear Lamins
- Lamins
Lamins are fibrous proteins having structural function in the cell nucleus.
In metazoan cells there are A and B type lamins which differ in their length and pI. Human cells have three differentially regulated genes. B-type lamins are present in every cell. B type lamins, B1 and B2, are expressed from the LMNB1 and LMNB2 genes on 5q23 and 19q13, respectively. A-type lamins are only expressed following gastrulation. Lamin A and C are the most common A-type lamins and are splice variants of the LMNA gene found at 1q21.
These proteins localize to two regions of the nuclear compartment, the nuclear lamina -- a proteinaceous structure layer subjacent to the inner surface of the nuclear envelope and throughout the nucleoplasm in the nucleoplasmic "veil".
Comparison of the lamins to vertebrate cytoskeletal IFs shows that lamins have an extra 42 residues (six heptads) within coil 1b. The c-terminal tail domain contains a nuclear localization signal (NLS), an Ig-fold like domain, and in most cases a carboxy-terminal CaaX box that is isoprenylated and carboxymethylated (lamin C does not have a CAAX box). Lamin A is further processed to remove the last 15 amino acids and its farnesylated cysteine.
During mitosis, lamins are phosphorylated by MPF which drives the disassembly of the lamina and the nuclear envleope.
Unclassified
phakinin philensin
Cell adhesion
At the plasma membrane, some keratins interact with desmosomes (cell-cell adhesion) and hemidesmosomes (cell-matrix adhesion) via adapter proteins.
Associated proteins
Filaggrin binds to keratin fibers in epidermal cells. Plectin links vimentin to other vimentin fibers, as well as to microfilaments, microtubules, and myosin II.
Keratin filaments in epithelial cells link to desmosomes(desmosomes connect the cytoskelton together) through plakoglobin, desmoplakin, desmogleins and desmocollins; desmin filaments are connected in a similar way in heart muscle cells.
Diseases arising from mutations in IF genes
- Epidermolysis bullosa simplex; K5 or K14 mutation
- Laminopathies are a family of diseases caused by mutations in nuclear lamins and include Hutchinson Gilford Progeria Syndrome and various lipodystrophies and cardiomyopathies among others.
- Human Intermediate Filament Database(HIFD), a comprehensive database of human intermediate filament proteins, their associated variations and diseases.
External links
|
||||||||||||||||||||