Thalamus
| Brain: Thalamus | ||
|---|---|---|
 |
||
| MRI cross-section of human brain, with thalamus marked. | ||
 |
||
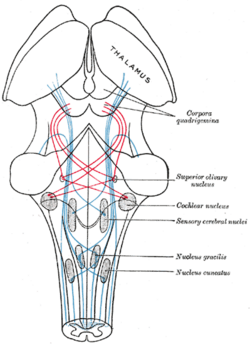
| Scheme showing the course of the fibers of the lemniscus; medial lemniscus in blue, lateral in red. | ||
| Latin | thalamus dorsalis | |
| Gray's | subject #189 808 | |
| NeuroNames | hier-283 | |
| MeSH | Thalamus | |
The thalamus (from Greek θάλαμος = room, chamber, IPA= /ˈθæləməs/) is a pair and symmetric part of the brain. It constitutes the main part of the diencephalon.
Contents |
Location and topography
In the caudal (tail) to rostral (head) sequence of neuromeres, the diencephalon is located between the mesencephalon (cerebral peduncule, belonging to the brain stem) and the cerebrum. The diencephalon includes also the dorsally located epithalamus (essentially the habenula and annexes) and the perithalamus (prethalamus formerly described as ventral thalamus) containing the zona incerta and the "reticulate nucleus" (not the reticular, term of confusion). Due to their different ontogenetic origins, the epithalamus and the perithalamus are formally distinguished from the thalamus proper.
Phylogenetic modifications are such that this article essentially deals with the human thalamus and may differ in comparison with accounts in non-upper primate species. In normal humans, the two thalami are prominent bulb-shaped masses, about 5.7 cm in length, located obliquely (about 30°) and symmetrically on each side of the third ventricle. The two can adhere on a variable extent in 30% of humans. This adhesio interthalamica (interthalamic adhesion, or massa intermedia) does not contain interthalamic neural connection in human beings.
Anatomy
The thalamus comprises a system of lamellae (made up of myelinated fibers) separating different thalamic subparts. Other areas are defined by distinct clusters of neurons, such as the periventricular gray, the intralaminar elements, the "nucleus limitans", and others.[1] These latter structures, different in structure from the major part of the thalamus, have been grouped together into the allothalamus as opposed to the isothalamus.[2] This distinction simplifies the global description of the thalamus.
See also List of thalamic nuclei.
Arterial supply
The thalamus derives its blood supply from a number of arteries including polar and paramedian arteries, inferolateral (thalamogeniculate) arteries, and posterior (medial and lateral) choroidal arteries.[3] These are all branches of the posterior cerebral artery.
Function
The thalamus is known to have multiple functions. Deduced from the design of the isothalamus, it is generally believed to act as a translator for which various "prethalamic" inputs are processed into a form readable by the cerebral cortex. The thalamus is believed to both process and relay sensory information selectively to various parts of the cerebral cortex, as one thalamic point may reach one or several regions in the cortex.
The thalamus also plays an important role in regulating states of sleep and wakefulness.[4] Thalamic nuclei have strong reciprocal connections with the cerebral cortex, forming thalamo-cortico-thalamic circuits that are believed to be involved with consciousness. The thalamus plays a major role in regulating arousal, the level of awareness, and activity. Damage to the thalamus can lead to permanent coma.
Many different functions are linked to the system to which thalamic parts belong. This is at first the case for sensory systems (which excepts the olfactory function) auditory, somatic, visceral, gustatory and visual systems where localised lesions provoke particular sensory deficits. A major role of the thalamus is devoted to "motor" systems. This has been and continues to be a subject of interest for investigators. VIm, the relay of cerebellar afferences, is the target of stereotactians particularly for the improvement of tremor. The role of the thalamus in the more anterior pallidal and nigral territories in the basal ganglia system disturbances is recognized but still poorly known. The contribution of the thalamus to vestibular or to tectal functions is almost ignored. The thalamus has been thought of as a "relay" that simply forwards signals to the cerebral cortex. Newer research suggests that thalamic function is more complicated.[5]
Pathology
Cerebrovascular accidents (strokes) can cause thalamic syndrome,[6] which results in a contralateral hemianaesthesia, burning or aching sensation on one half of a body (painful anaesthesia) often accompanied by mood swings. Ischemia of the territory of the paramedian artery, if bilateral, causes serious troubles including akinetic mutism accompanied or not by oculomotor troubles. It is also related to Thalamocortical Dysrhythmia.
Korsakoff's Syndrome stems from mammillary bodies, mammilothalamic, or thalamic lesions.
Development
The thalamic complex is composed of the perithalamus (or prethalamus, previously also known as ventral thalamus), the zona limitans intrathalamica (ZLI) and the thalamus (dorsal thalamus).[7][8]
The ZLI is a transverse boundary located between the perithalamus and the functional distinct thalamus. Besides its morphological characteristics, it bears the hallmarks of a signalling centre. Fate mapping experiments in chicks have shown that the ZLI is cell lineage restricted at its boundaries and therefore can be termed a true developmental compartment in the forebrain.[9]
Besides morphological characteristics, the ZLI is the only structure in the alar plate of the neural tube that expresses signaling molecules.[10]
In mice, the function of signaling at the ZLI has not been addressed directly due to a complete absence of the diencephalon in Shh mutants.[11]
Studies in chicks have shown that Shh is both necessary and sufficient for thalamic gene induction.[12]
In zebrafish, it was shown that the expression of two Shh genes, shh-a and shh-b (formerly described as twhh) mark the ZLI territory, and that Shh signaling is sufficient for the molecular differentiation of both the prethalamus and the thalamus but is not required for their maintenance and Shh signaling from the ZLI/alar plate is sufficient for the maturation of prethalamic and thalamic territory while ventral Shh signals are dispensable.[13]
In humans, a common genetic variation in the promotor region of the serotonin transporter (the SERT-long and -short allele: 5-HTTLPR) has been shown to affect the development of several regions of the thalamus in adults. People who inherit two short alleles (SERT-ss) have more neurons and a larger volume in the pulvinar and possibly the limbic regions of the thalamus. Enlargement of the thalamus provides an anatomical basis for why people who inherit two SERT-ss alleles are more vulnerable to major depression, posttraumatic stress disorder, and suicide.[14]
References
- ↑ Jones Edward G.(2007) "The Thalamus" Cambridge Uni. Press
- ↑ Percheron, G. (2003) "Thalamus". In Paxinos, G. and May, J.(eds). The human nervous system. 2d Ed. Elsevier. Amsterdam. pp.592-675
- ↑ Percheron, G. (1982) The arterial supply of the thalamus. In Schaltenbrand and Walker, A.E.(eds) Stereotaxy of the human brain. Thieme . Stuttgart. pp.218-232
- ↑ Steriade, M. and Llinas, R. (1988) "The functional states of the thalamus and the associated neuronal interplay". Physiological Reviews 68: 699-742
- ↑ Your Brain Boots Up Like a Computer | LiveScience
- ↑ Dejerine, J. and Roussy. G.(1906) Le syndrome thalamique. Rev. Neurol. 14: 521-532
- ↑ Kuhlenbeck, H. (1937). The ontogenetic development of diencephalic centres in the bird's brain (chick) and comparison with the reptilian and mammalian diencephalon. J. Comp. Neurol. 66
- ↑ Shimamura, K., Hartigan, D. J., Martinez, S., Puelles, L. and Rubenstein, J. L. (1995). Longitudinal organization of the anterior neural plate and neural tube. Development 121,3923 -3933.
- ↑ Zeltser, L. M., Larsen, C. W. and Lumsden, A. (2001). A new developmental compartment in the forebrain regulated by Lunatic fringe. Nat. Neurosci. 4, 683-684.
- ↑ Puelles, L. and Rubenstein, J. L. (2003). Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 26,469 -476.
- ↑ Ishibashi, M. and McMahon, A. P. (2002). A sonic hedgehog-dependent signalling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development 129,4807 -4819.
- ↑ Kiecker, C. and Lumsden, A. (2004). Hedgehog signalling from the ZLI regulates diencephalic regional identity. Nat. Neurosci. 7,1242 -1249.
- ↑ Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon'. Development. 2006 Mar;133(5):855-64[1]
- ↑ Young KA, Holcomb LA, Yazdani U, Bonkale W, Hicks PB and German DC (2007). "5HTTLPR polymorphism and enlargement of the pulvinar: Unlocking the backdoor to the limbic system". Biol Psychiatry 61: 813–8. doi:. PMID 17083920.
See also
- Primate basal ganglia system
- Regions in the human thalamus
- Thalamus (non primate)
- List of thalamic nuclei
Additional images
External links
- http://www.scholarpedia.org/article/Thalamus
- BrainMaps at UCDavis thalamus
|
||||||||||||||