Haloperidol
 |
|
 |
|
|
Haloperidol
|
|
| Systematic (IUPAC) name | |
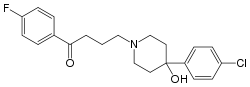
| 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]- 1-(4-fluorophenyl)-butan-1-one |
|
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C21H23ClFNO2 |
| Mol. mass | 375.9 g/mol (plain haloperidol) |
| Pharmacokinetic data | |
| Bioavailability | Approx. 60 to 70% (tablets and liquid) |
| Metabolism | hepatic |
| Half life | 12 to 36 hours |
| Excretion | Biliary and renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status |
℞ Prescription only |
| Routes | Oral, IM, IV, depot (as decanoate ester) |
Haloperidol is a typical antipsychotic. It is in the butyrophenone class of antipsychotic medications and has pharmacological effects similar to the phenothiazines.
Haloperidol is a primitive antipsychotic used in the treatment of schizophrenia and, more acutely, in the treatment of acute psychotic states and delirium. A long-acting decanoate ester is used as a long acting injection given every 4 weeks to people with schizophrenia or related illnesses who have a poor compliance with medication and suffer frequent relapses of illness. In some countries this can be involuntary under Community Treatment Orders.
It was developed in 1957 by the Belgian company Janssen Pharmaceutica and submitted to first clinical trials in Belgium in the same year[1]. After being rejected by U.S. company Searle due to side effects, it was later marketed in the U.S. by McNeil Laboratories. It was approved by the FDA on April 12, 1967.
Haloperidol is sold under the tradenames Aloperidin, Bioperidolo, Brotopon, Dozic, Duraperidol (Germany), Einalon S, Eukystol, Haldol, Halosten, Keselan, Linton, Peluces, Serenace, Serenase, and Sigaperidol. In medical slang, haloperidol is occasionally called vitamin H.[2]
Contents |
Pharmacology
Haloperidol is a neuroleptic and a butyrophenone. Due to its strong central antidopaminergic action, it is classified as a highly potent neuroleptic. It is approximately 50 times more potent than chlorpromazine on a weight basis (50mg chlorpromazine are equivalent to 1mg haloperidol). Haloperidol possesses a strong activity against delusions and hallucinations, most likely due to an effective dopaminergic receptor blockage in the mesocortex and the limbic system of the brain. It blocks the dopaminergic action in the nigrostriatal pathways, which is the probable reason for the high frequency of extrapyramidal-motoric side-effects (dystonias, akathisia, pseudoparkinsonism). It has minor antihistaminic and anticholinergic properties, therefore cardiovascular and anticholinergic side-effects such as hypotension, dry mouth, constipation, etc., are seen quite infrequently, compared with less potent neuroleptics such as chlorpromazine. Haloperidol also has sedative properties and displays a strong action against psychomotor agitation but due to a specific action in the limbic system. It therefore is an effective treatment for mania and states of agitation. Additionally, it can be given as an adjuvant in the therapy of severe chronic pain.
The peripheral antidopaminergic effects of haloperidol account for its strong antiemetic activity. There, it acts at the chemoreceptor trigger zone (CTZ). Haloperidol is useful to treat severe forms of nausea/emesis such as those resulting from chemotherapy. The peripheral effects lead also to a relaxation of the gastric sphincter muscle and an increased release of the hormone prolactin, with the possible emergence of breast enlargement and secretion of milk (lactation) in both sexes.
Pharmacokinetics
Intramuscular injections
The drug is well and rapidly absorbed and has a high bioavailability. Plasma-levels reach their maximum within 20 minutes after injection. The decanoate injectable formulation is for intramuscular administration only and should never be used intravenously.
Intravenous injections
The bioavailability is 100% and the very rapid onset of action is seen within about ten minutes. The duration of action is 3 to 6 hours. If haloperidol is given as slow IV infusion, the onset of action is retarded, but the duration prolonged compared to IM injection.
Therapeutic concentrations
Plasma levels of 4 micrograms per liter to 20 (up to 25) micrograms per liter are required for therapeutic action. The determination of plasma levels can be used to calculate dose adjustments and to check compliance, particularly in long-term patients. Plasma levels in excess of the therapeutic range may lead to a higher incidence of side-effects or even pose the risk of haloperidol intoxication.
Uses
A comprehensive review of haloperidol has found it to be an effective agent in treatment of symptoms associated with schizophrenia.[3] Haloperidol is also used in the control of the symptoms of:
- Acute psychosis, such as drug psychosis (LSD, psilocybin, amphetamines, ketamine,[4] and phencyclidine [5]), psychosis associated with high fever or metabolic disease
- Acute manic phases until the concomitantly given first-line drugs such as lithium or valproate are effective
- Hyperactivity, aggression.
- Acute delirium
- Otherwise uncontrollable severe behavioral disorders in children and adolescents
- Agitation and confusion associated with cerebral sclerosis
- Adjunctive treatment of alcohol and opioid withdrawal
- Treatment of neurological disorders such as tic disorders, Tourette syndrome, and chorea
- Treatment of severe nausea/emesis (postoperative, side-effects of radiation and cancer chemotherapy)
- Adjunctive treatment of severe chronic pain, always together with analgesics
- Therapeutic trial in personality disorders such as borderline personality disorders
- Also used in the treatment of Intractable hiccups
Some weeks or even months of treatment may be needed before a remission of schizophrenia is evident.
In some clinics the use of atypical neuroleptics (e.g. clozapine, risperidone, olanzapine, ziprasidone) is generally preferred over haloperidol, because these drugs have an appreciably lower incidence of extrapyramidal side-effects. Each of these drugs, however, has its own spectrum of potentially serious side-effects (e.g. agranulocytosis with clozapine, weight gain with increased risk of diabetes and of stroke). Atypical neuroleptics are also much more expensive and have recently been the subject of increasing controversy regarding their efficacy in comparison to older products and side effects.
Haloperidol was considered indispensable for treating psychiatric emergency situations,[6][7] although the newer atypical drugs have gained greater role in a number of situations as outlined in a series of consensus reviews published between 2001 and 2005.[8][9][10][3] It is enrolled in the World Health Organization List of Essential Medicines.
As is common with typical neuroleptics, haloperidol is by far more active against "positive" psychotic symptoms (delusions, hallucinations etc.) than against "negative" symptoms (social withdrawal, autism etc.). With the exception of the highly effective clozapine, the effectiveness of haloperidol against positive symptoms has not been outperformed by newer antipsychotics.
A multi-year UK study by the Alzheimer's Research Trust suggested that this and other neuroleptic anti-psychotic drugs commonly given to Alzheimer's patients with mild behavioural problems often make their condition worse. [11] The study concluded that
| “ | For most patients with AD, withdrawal of neuroleptics had no overall detrimental effect on functional and cognitive status and by some measures improved functional and cognitive status. Neuroleptics may have some value in the maintenance treatment of more severe neuropsychiatric symptoms, but this possibility must be weighed against the unwanted effects of therapy. The current study helps to inform a clinical management strategy for current practice, but the considerable risks of maintenance therapy highlight the urgency of further work to find, develop, and implement safer and more effective treatment approaches for neuropsychiatric symptoms in people with AD. | ” |
The television show Twin Peaks includes an instance in which Haloperidol is used during an interrogation as a truth serum. There is no recorded history of Haloperidol actually being used for this purpose, and many of the effects on the show seem to be the opposite of the drugs actual effects (including the induction of a psychotic state).
Contraindications
Absolute
- Preexisting coma, acute stroke
- Severe intoxication with alcohol or other central depressant drugs
- Known allergy against haloperidol or other butyrophenones or other drug ingredients
- Known heart disease; when combined will tend towards cardiac arrest
Special caution needed
- Preexisting Parkinson's disease[13]
- Patients at special risk for the development of QT prolongation (hypokalemia, concomitant use of other drugs causing QT prolongation)
- Compromised liver-function (as haloperidol is metabolized and eliminated mainly by the liver, dose reductions and/or spaced intervals may be needed)
- Haloperidol may decrease the seizure-threshold. Treat patients with epilepsy and those with risk factors for the development of seizures (alcohol withdrawal, encephalopathy) with caution. Maintain existing anticonvulsive therapy.
- Patients with hyperthyreosis; the action of haloperidol is intensified and side-effects are more likely. Initiate an effective therapy of hyperthyreosis.
- IV injections: inject slowly to avoid hypotension or orthostatic collapse. Avoid IV injections in cardiovascular unstable patients (preexisting hypotension, shock, concomitant antihypertensive therapy, heart insufficiency). Prefer in these cases moderate oral or IM doses.
Adverse effects
The drug is noted for its strong early and late extrapyramidal side-effects.[3] The risk of the facial disfiguring tardive dyskinesia is around 4% per year in younger patients, higher than with most other antipsychotic drugs. In patients over the age of 45, the percentage of those afflicted can be even higher. Other predispositive factors may be female gender, preexisting affective disorder and cerebral dysfunction.
Akathisia manifests itself with anxiety, dysphoria, and an inability to remain motionless.
Other side effects include dry mouth, lethargy, restlessness of akathisia, muscle-stiffness, muscle-cramping, restlessness, tremors, and weight-gain; side effects like these are more likely to occur when the drug is given in high doses and/or during long-term treatment. Depression, severe enough to result in suicide, is quite often seen during long-term treatment. Care should be taken to detect and treat depression early in course. Sometimes the change from haloperidol to a mildly potent neuroleptic (e.g. chlorprothixene or chlorpromazine), together with appropriate antidepressant therapy, does help. Sedative and anticholinergic side-effects occur more frequently in the elderly.
The potentially fatal neuroleptic malignant syndrome (NMS) is a significant possible side effect. Haloperidol and fluphenazine are the two drugs which cause NMS most often. NMS involves fever and other symptoms. Allergic and toxic side-effects are uncommon. Skin rash and photosensitivity both occur in less than 1% of patients.
Children and adolescents are particularly sensitive to the early and late extrapyramidal side-effects of haloperidol. It is recommended to treat pediatric patients only if clearly needed and if the psychiatric or neurologic disorder is substantial.
QT prolongation with sudden death is rarely seen. Likewise, the development of thromboembolic complications are also rare.
Haloperidol may have a negative impact on vigilance or decrease the ability of the patient to drive or operate a machine, particularly initially.
Haloperidol is completely devoid of any potential psychological dependence. A greater problem is that psychiatric patients prescribed this drug may seek to avoid taking it and consequently risk relapse of their symptoms.
Unpleasant withdrawal symptoms, if haloperidol is stopped abruptly after long-term treatment, are sometimes noted. These are usually agitation, anxiety, insomnia, and nausea. But agitation is also a side effect of this drug because of akathisia. Rebound of psychotic symptoms and mood swing into mania are also seen.
Haloperidol has been shown to dramatically increase dopamine activity, up to 98%, in test subjects after two weeks on a "moderate to high" dose compared to chronic schizophrenics.[14] In another study, a live survey of a patient showed that the person has 90% more dopamine receptors, of the D2 subtype, than before treatment with haloperidol.[15] The long term effect of this is unknown, but the first study concludes that this upregulation is positively associated with severe dyskinesias.(more upregulation, more dyskinesia)
Other Remarks
During long-term treatment of chronic psychiatric disorders, it should be tried - in regular intervals - to reduce the daily dose to the lowest level needed for maintenance of remission. Sometimes, it may be indicated to terminate haloperidol treatment gradually.
Other forms of therapy (psychotherapy, occupational therapy/ergotherapie, social rehabilitation) should be instituted properly.
Pregnancy and lactation
Data from animal experiments indicate haloperidol is not teratogenic, but is embryotoxic in high doses. In humans, no controlled studies exist. Unconfirmed studies in pregnant women revealed possible damage to the fetus, although most of the women were exposed to multiple drugs during pregnancy. Following accepted general principles, haloperidol should only be given during pregnancy if the benefit to the mother clearly outweighs the potential fetal risk.
Haloperidol, when given to lactating women, is found in significant amounts in their milk. Breastfed children sometimes show extrapyramidal symptoms. If the use of haloperidol during lactation seems indicated, the benefit for the mother should clearly outweigh the risk for the child. Consider termination of breastfeeding.
Carcinogenicity
So far, no statistically acceptable evidence is found to associate long-term use of haloperidol with the potential for increased breast cancer risk in female patients. In an unconfirmed study, relative risks of breast cancer, in inmates of the Buffalo Psychiatric Center undergoing long-term treatment with haloperidol, were 3.5 (compared to patients hospitalized in general or internal medicine facilities) and 9.5 (general population), respectively.[16] These results need confirmation by larger studies. If true, carcinogenity is most probably related to the strong increase in plasma-levels of prolactin under long-term treatment with haloperidol. This news is another good reason to avoid any unnecessary use of haloperidol.
Interactions
- Other central depressants (alcohol, tranquilizers, narcotics): actions and side-effects of these drugs (sedation, respiratory depression) are increased. In particular, the doses of concomitantly used opioids for chronic pain can be reduced by 50%.
- Methyldopa: increased risk of extrapyramidal side-effects and other unwanted central effects
- Levodopa: decreased action of levodopa
- Tricyclic antidepressants: metabolism and elimination of tricyclics significantly decreased, increased toxicity noted (anticholinergic and cardiovascular side-effects, lowering of seizure-threshold)
- Quinidine, buspirone, and fluoxetine: increased plasma-levels of haloperidol, decrease haloperidol dose, if necessary
- Carbamazepine, phenobarbital, and rifampicin: plasma-levels of haloperidol significantly decreased, increase haloperidol dose, if necessary.
- lithium: rare cases of the following symptoms have been noted: encephalopathy, early and late extrapyramidal side-effects, other neurologic symptoms and coma. Check lithium plasma levels regularly and keep the dose of haloperidol as low as possible.
- Guanethidine: antihypertensive action antagonized
- Epinephrine: action antagonized, paradoxical decrease in blood pressure may result
Doses
As directed by the physician, depends on the condition to be treated, age and weight of patient:
- Acute problems: single doses of 1 mg to 5 mg (up to 10mg) oral or i.m., usually repeated every 4 to 8 hours. Do not exceed an oral dose of 100 mg daily. Doses used for IV injection are usually 5 to 10 mg as a single dose; not exceeding 50 mg daily.
- Chronic conditions: 0.5 to 20 mg daily oral, rarely more. The lowest dose that maintains remission should be employed.
- Experimental doses: In resistant cases of psychosis small studies with oral doses of up to 300 mg - 500 mg daily have been conducted (in most cases together with an anticholinergic antiparkinsonian drug (Biperiden, Benzatropine, etc.) to avoid severe early extrapyramidal side-effects. These studies showed no superior results and led to severe side-effects. Also, the frequency of otherwise unusual side-effects (hypotension, QT-time prolongation, and serious cardiac arrhythmias) was dramatically increased. The clinical use of haloperidol in these doses is discouraged now and it is recommended to switch the patient gradually to a different neuroleptic (e.g. clozapine, olanzapine, aripiprazole).
Depot forms are also available; these are injected deeply i.m. at regular intervals. The depot forms are not suitable for initial treatment
Overdose
Experimental evidence from animal studies indicates that doses needed for acute poisoning are quite high in relation to therapeutic doses.
Overdoses with depot injections are uncommon, because almost always experienced personnel administer them to patients.
Symptoms
Symptoms are usually due to exaggerated side-effects. Most often encountered are:
- Severe extrapyramidal side-effects with muscle rigidity and tremors, akathisia etc. (inject 5 mg biperiden (Akineton) slowly IV, repeat after some hours if necessary). Sometimes oral or IM treatment with biperiden is needed for several days or even weeks. If the patient is very upset about extrapyramidal side-effects, small doses of lorazepam (0.5 to 1 mg orally, repeated every 4 to 6 hours if necessary) can be given. Lorazepam has an intrinsic action against upset, anxiety, and extrapyramidal side-effects.
- Hypotension or hypertension
- Sedation
- Anticholinergic side-effects (dry mouth, constipation, paralytic ileus, difficulties in urinating, massive sweating). Cautious doses of physostigmine may be given repeatedly. Physostigmine may increase the risk of seizures.
- Coma in severe cases, accompanied by respiratory depression and massive hypotension, shock
- Rarely serious ventricular arrhythmia (torsades de pointes) with or without prolonged QT-time
- Epileptic seizures, give careful doses of diazepam 5 mg to 10 mg by slow IV injection, repeatedly if needed, until seizures subside. Take care not to worsen central depression or respiratory depression caused by haloperidol. The treatment facility should be able to institute artificial respiration readily. Valproate first given as slow IV infusion and later orally may also be effective.
Treatment
Treatment is merely symptomatic and involves intensive care with stabilization of vital functions. In early detected cases of oral overdose induction of emesis, gastric lavage and the use of activated charcoal can all be tried. Avoid epinephrine for treatment of hypotension and shock, because its action might be reversed.
Prognosis
Generally, the prognosis of overdose is good and lasting damage is not known, provided that the patient has survived the initial phase.
Other formulations
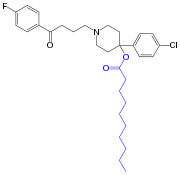
The decanoate ester of haloperidol (Haloperidol decanoate, trade names Haldol decanoate, Halomonth, Neoperidole) has a much longer duration of action, and therefore is used in noncompliant people. A dose of 25 to 250 mg is given by intramuscular injection once every two to four weeks.[17]
The IUPAC name of haloperidol decanoate is 4-(4-chlorophenyl)-1-1[4-(4-fluorophenyl)-4-oxobutyl]-4 piperidinyl decanoate.
Veterinary use
Haloperidol is also used on many different kinds of animals. It appears to be particularly successful when given to birds; e.g. a parrot that will not otherwise stop plucking its feathers out.
Dose forms
- Liquid: 2 mg/mL, also 10 mg/mL
- Tablets: 0.5 mg, 1 mg, 2 mg, 5 mg, 10 mg, 20 mg
- Injection: 5 mg (1 mL)
- Depot injection forms
- The original drug Haldol and many generics are available
See also
References
- ↑ B. Granger, S. Albu, The Haloperidol Story, Annals of Clinical Psychiatry (after Jan 1, 2004), Volume 17, Number 3, Number 3/July-September 2005 , pp. 137-140(4)
- ↑ "Medical Jargon". Retrieved on 2008-09-21.
- ↑ 3.0 3.1 3.2 Joy CB, Adams CE, Lawrie SM (2006). "Haloperidol versus placebo for schizophrenia". Cochrane Database Syst Rev (4): CD003082. doi:. PMID 17054159.
- ↑ AJ Giannini, N Underwood, M Condon. Acute ketamine intoxication treated by haloperidol. American Journal of Therapeutics.7:389-341,2000.
- ↑ AJ Giannini, WA Price. Antidotal strategies in phencyclidine intoxication. International Journal of Psychiatry in Medicine. 14(4):315-319.1984.
- ↑ Cavanaugh SV (1986). "Psychiatric emergencies". Med. Clin. North Am. 70 (5): 1185–202. PMID 3736271.
- ↑ Currier GW (2003). "The controversy over "chemical restraint" in acute care psychiatry". J Psychiatr Pract 9 (1): 59–70. doi:. PMID 15985915.
- ↑ Allen MH, Currier GW, Hughes DH, Reyes-Harde M, Docherty JP (2001). "The Expert Consensus Guideline Series. Treatment of behavioral emergencies". Postgrad Med (Spec No): 1–88; quiz 89–90. PMID 11500996.
- ↑ Allen MH, Currier GW, Hughes DH, Docherty JP, Carpenter D, Ross R (2003). "Treatment of behavioral emergencies: a summary of the expert consensus guidelines". J Psychiatr Pract 9 (1): 16–38. doi:. PMID 15985913.
- ↑ Allen MH, Currier GW, Carpenter D, Ross RW, Docherty JP (2005). "The expert consensus guideline series. Treatment of behavioral emergencies 2005". J Psychiatr Pract 11 Suppl 1: 5–108; quiz 110–2. doi:. PMID 16319571.
- ↑ "Medication 'worsens Alzheimer's'", BBC News (1 April 2008). Retrieved on 2008-04-01. "Neuroleptics provided no benefit for patients with mild behavioural problems, but were associated with a marked deterioration in verbal skills."
- ↑ Ballard C, Lana MM, Theodoulou M, Douglas S, McShane R, et al. (2008). "A Randomised, Blinded, Placebo-Controlled Trial in Dementia Patients Continuing or Stopping Neuroleptics (The DART-AD Trial)". PLOS Medicine 5 (4, e76): e76. doi:.
- ↑ ""Delirium in Elderly People: An Update" - Medscape". Retrieved on 2008-02-21.
- ↑ Silvestri S, Seeman MV, Negrete JC, et al (October 2000). "Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study". Psychopharmacology 152 (2): 174–80. doi:. PMID 11057521.
- ↑ Silvestri S, Seeman MV, Negrete JC, et al (October 2000). "Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study". Psychopharmacology 152 (2): 174–80. doi:. PMID 11057521.
- ↑ Halbreich U, Shen J, Panaro V (1996). "Are chronic psychiatric patients at increased risk for developing breast cancer?". Am J Psychiatry 153 (4): 559–60. PMID 8599407. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=8599407.
- ↑ Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th edition (McGraw-Hill, 2001).
External links
- Rx-List.com - Haloperidol
- Medline plus - Haloperidol
- Swiss scientific information on Haldol
- "WHO List of Essential Drugs". Archived from the original on 2007-12-29.
- German antipsychiatry comments regarding cancerogenity
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||