Haemophilia
| Hemophilia Classification and external resources |
|
| ICD-10 | D66.-D68. |
|---|---|
| ICD-9 | 286 |
| OMIM | 306700 306900 264900 |
| DiseasesDB | 5555 5561 29376 |
| MedlinePlus | 000537 |
| eMedicine | med/3528 |
| MeSH | D025861 |
Haemophilia (also spelled as hemophilia, from the Greek haima "blood" and philia "to love"[1]) is a group of hereditary genetic disorders that impair the body's ability to control blood clotting or coagulation. In its most common form, Hemophilia A, clotting factor VIII is absent. In Haemophilia B, factor IX is deficient. Hemophilia A occurs in about 1 in 5,000–10,000 male births[2], while Hemophilia B occurs at about 1 in about 20,000–34,000.
The effects of this sex-linked, X chromosome disorder are manifested almost entirely in males, although the gene for the disorder is inherited from the mother. Females have two X chromosomes while males have only one, lacking a 'back up' copy for the defective gene. Females are therefore almost exclusively carriers of the disorder, and may have inherited it from either their mother or father.
These genetic deficiencies may lower blood plasma clotting factor levels of coagulation factors needed for a normal clotting process. When a blood vessel is injured, a temporary scab does form, but the missing coagulation factors prevent fibrin formation which is necessary to maintain the blood clot. Thus a haemophiliac does not bleed more intensely than a normal person, but for a much longer amount of time. In severe haemophiliacs even a minor injury could result in blood loss lasting days, weeks, or not ever healing completely. The critical risk here is with normally small injuries which, due to missing factor VIII, take long times to heal. In areas such as the brain or inside joints this can be fatal or permanently debilitating.
The bleeding with external injury is normal, but incidence of late re-bleeding and internal bleeding is increased, especially into muscles, joints, or bleeding into closed spaces. Major complications include hemarthrosis, hemorrhage, gastrointestinal bleeding, and menorrhagia.
Contents |
Causes

Hemophilia is nearly always caused by a genetic error causing the lack of a normally functioning clotting factor. While these disorders are typically congenital and present from birth, in rare cases, they may be acquired at some point later in life due to various health complications.
- Hemophilia A is an X-linked genetic disorder involving a lack of functional clotting Factor VIII and represents 90% of haemophilia cases.[3]
- Hemophilia B is an X-linked genetic disorder involving a lack of functional clotting Factor IX It is less Severe but more uncommon than Hemophilia A.
- Hemophilia C is an autosomal recessive genetic disorder involving a lack of functional clotting Factor XI.
Occurrence
Hemophilia is quite rare, with only about 1 instance in every 10,000 births (or 1 in 5,001 male births) for hemophilia A and 1 in 50,000 births for hemophilia B.[4] About 18,000 people in the United States have hemophilia. Each year in the US, about 400 babies are born with the disorder. Hemophilia usually occurs in males and less often in females.[5] It is estimated that about 2500 Canadians have hemophilia A and about 500 Canadians have hemophilia B. [6]
History

The earliest possible implicit reference to hemophilia may have been in the Talmud[7], a Jewish holy text, which states that males did not have to be circumcised if two brothers had already died from the procedure. In 1000 CE, the Arab physician, Abu al-Qasim al-Zahrawi (known as Albucasis in the West), wrote the first explicit description of hemophilia in his Al-Tasrif, in which he wrote of an Andalusian family whose males died of bleeding after minor injuries.[8]
In 1803, Dr. John Conrad Otto, a Philadelphia physician, wrote an account about "a hemorrhagic disposition existing in certain families." He recognized that the disorder was hereditary and that it affected males and rarely females. He was able to trace the disease back to a woman who settled near Plymouth in 1720. The first usage of the term "hemophilia" appears in a description of the condition written by Hopff at the University of Zurich in 1828.[9] In 1937, Patek and Taylor, two doctors from Harvard, discovered anti-hemophilic globulin. Pavlosky, a doctor from Buenos Aires, found Hemophilia A and Hemophilia B to be separate diseases by doing a lab test. This test was done by transferring the blood of one hemophiliac to another hemophiliac. The fact that this corrected the clotting problem showed that there was more than one form of hemophilia.
Haemophilia in European royalty featured prominently and thus is sometimes known as "the royal disease". Queen Victoria passed the mutation to her son Leopold and, through several of her daughters, to various royals across the continent, including the royal families of Spain, Germany, and Russia. Tsarevich Alexei Nikolaevich, son of Nicholas II, was a descendant of Queen Victoria and suffered from hemophilia. It was claimed that Rasputin was successful at treating the Tsarevich Alexei of Russia's hemophilia. At the time, a common treatment administered by professional doctors was to use aspirin, which worsened rather than lessen the problem. It is believed that, by simply advising against the medical treatment, Rasputin could bring visible and significant improvement to the condition of Alexei.
Prior to 1985, there were no laws enacted within the U.S. to screen blood. As a result, many hemophilia patients who received untested and unscreened clotting factor prior to 1992 were at an extreme risk for contracting HIV and Hepatitis C via these blood products. It is estimated that more than 50% of the Hemophilia population, over 10,000 people, contracted HIV from the tainted blood supply in the United States alone.[10]
As a direct result of the contamination of the blood supply in the late 1970s and early/mid 1980s with viruses such as Hepatitis and HIV, new methods were developed in the production of clotting factor products. The initial response was to heat-treat (pasteurize) plasma-derived factor concentrate, followed by the development of monoclonal factor concentrates, which use a combination of heat treatment and affinity chromatography to inactivate any viral agents in the pooled plasma from which the factor concentrate is derived. The Lindsay Tribunal in Ireland investigated, among other things, the slow adoption of the new methods.
Genetics
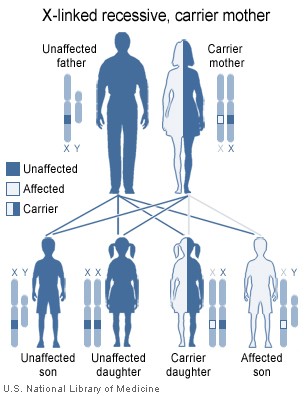
Females possess two X-chromosomes, whereas males have one X and one Y chromosome. Since the mutations causing the disease are recessive, a woman carrying the defect on one of her X-chromosomes may not be affected by it, as the equivalent allele on her other chromosome should express itself to produce the necessary clotting factors. However the Y-chromosome in men has no gene for factors VIII or IX. If the genes responsible for production of factor VIII or factor IX present on a male's X-chromosome are deficient there is no equivalent on the Y-chromosome, so the deficient gene is not masked by the dominant allele and he will develop the illness.
Since a male receives his single X-chromosome from his mother, the son of a healthy female silently carrying the deficient gene will have a 50% chance of inheriting that gene from her and with it the disease; and if his mother is affected with haemophilia, he will have a 100% chance of being a haemophiliac. In contrast, for a female to inherit the disease, she must receive two deficient X-chromosomes, one from her mother and the other from her father (who must therefore be a haemophiliac himself). Hence haemophilia is far more common among males than females. However it is possible for female carriers to become mild haemophiliacs due to lyonisation (inactivation) of the X chromosomes. Haemophiliac daughters are more common than they once were, as improved treatments for the disease have allowed more haemophiliac males to survive to adulthood and become parents. Adult females may experience menorrhagia (heavy periods) due to the bleeding tendency. The pattern of inheritance is criss-cross type. This type of pattern is also seen in colour blindness.
A mother who is a carrier has a 50% chance of passing the faulty X chromosome to her daughter, while an affected father will always pass on the affected gene to his daughters. A son cannot inherit the defective gene from his father.
Genetic testing and genetic counseling is recommended for families with haemophilia. Prenatal testing, such as amniocentesis, is available to pregnant women who may be carriers of the condition.
As with all genetic disorders, it is of course also possible for a human to acquire it spontaneously through mutation, rather than inheriting it, because of a new mutation in one of their parents' gametes. Spontaneous mutations account for about 33% of all cases of haemophilia A. About 30% of cases of Hemophilia B are the result of a spontaneous gene mutation[2].
Probability
If a female gives birth to a haemophiliac child, either the female is a carrier for the disease or the haemophilia was the result of a spontaneous mutation. Until modern direct DNA testing, however, it was impossible to determine if a female with only healthy children was a carrier or not. Generally, the more healthy sons she bore, the higher the probability that she was not a carrier.
If a male is afflicted with the disease and has children with a female who is not even a carrier, his daughters will be carriers of haemophilia. His sons, however, will not be affected with the disease. This is because the disease is X-linked and the father cannot pass haemophilia through the Y chromosome. Males with the disorder are then no more likely to pass on the gene to their children than carrier females, though all daughter they sire will be carriers and all sons they father will not have hemophilia (unless the mother is a carrier).
Treatment
Though there is no cure for hemophilia, it can be controlled with regular infusions of the deficient clotting factor, i.e. factor VIII in haemophilia A or factor IX in hemophilia B. Factor replacement can be either isolated from human blood serum, recombinant, or a combination of the two. Some hemophiliacs develop antibodies (inhibitors) against the replacement factors given to them, so the amount of the factor has to be increased or non-human replacement products must be given, such as porcine factor VIII.
If a patient becomes refractory to replacement coagulation factor as a result of circulating inhibitors, this may be partially overcome with recombinant human factor VII (NovoSeven), which is registered for this indication in many countries.
In early 2008, the US Food and Drug Administration approved Xyntha (Wyeth) anti-hemophilic factor, genetically engineered from the genes of Chinese hamster ovary cells. Since 1993 (Dr. Mary Nugent) recombinant factor products (which are typically cultured in Chinese hamster ovary (CHO) tissue culture cells and involve little, if any human plasma products) have been available and have been widely used in wealthier western countries. While recombinant clotting factor products offer higher purity and safety, they are, like concentrate, extremely expensive, and not generally available in the developing world. In many cases, factor products of any sort are difficult to obtain in developing countries.
In Western countries, common standards of care fall into one of two categories: prophylaxis or on-demand. Prophylaxis involves the infusion of clotting factor on a regular schedule in order to keep clotting levels sufficiently high to prevent spontaneous bleeding episodes. On-demand treatment involves treating bleeding episodes once they arise. In 2007, a clinical trial was published in the New England Journal of Medicine (NEJM) comparing on-demand treatment of boys (< 30 months) with Hemophilia A with prophylactic treatment (infusions of 25 IU/kg body weight of Factor VIII every other day) in respect to its effect on the prevention of joint-diseases. When the boys reached 6 years of age, 93% of those in the prophylaxis group and 55% of those in the episodic-therapy group had a normal index joint-structure on MRI. [11] Prophylactic treatment, however, resulted in average costs of $300,000 per year. The author of an editorial published in the same issue of the NEJM demands more clinical studies addressing the cost-effectiveness of prophylactic treatment. [12]
It is recommended that people affected with Hemophilia do specific exercises to strengthen the joints, particularly the elbows, knees, and ankles.[13] Exercises include elements which increase flexibility, tone, and strength of muscles, increasing their ability to protect joints from damaging bleeds. These exercises are recommended after an internal bleed occurs and on a daily basis to strengthen the muscles and joints to prevent new bleeding problems. Many recommended exercises include standard sports warm-up and training exercises such as stretching of the calves, ankle circles, elbow flexions, and Quadriceps sets.
Alternative and complementary treatments
Scientific studies indicate that hypnosis and self-hypnosis can be effective at reducing bleeds and the severity of bleeds and thus the frequency of factor treatment.[14] Herbs which strengthen blood vessels and act as astringents may also benefit patients with hemophilia, however there is little or no peer reviewed research to support these claims. Recommended herbs include: Bilberry(Vaccinium myrtillus), Grape seed extract (Vitis vinifera), Scotch broom (Cytisus scoparius), Stinging nettle (Urtica dioica), Witch hazel (Hamamelis virginiana), and yarrow (Achillea millefolium).[14]
Differential diagnosis
Haemophilia A can be mimicked by von Willebrand disease
- von Willebrand Disease type 2A, where decreased levels of von Willebrand Factor can lead to premature proteolysis of Factor VIII. In contrast to haemophilia, vWD type 2A is inherited in an autosomal dominant fashion.
- von Willebrand Disease type 2N, where von Willebrand Factor cannot bind Factor VIII, autosomal recessive inheritance. (ie; both parents need to give the child a copy of the gene). [1]
- von Willebrand Disease type 3, where lack of von Willebrand Factor causes premature proteolysis of Factor VIII. In contrast to haemophilia, vWD type 3 is inherited in an autosomal recessive fashion.
Support group links
National and international advocacy and support organizations
- World Federation of Hemophilia (WFH)
- Canadian Hemophilia Society
- National Hemophilia Foundation
- Hemophilia Federation of America
- UK Haemophilia Society (charity)
- European Haemophilia Consortium (EHC)
- Haemophilia Foundation Australia (HFA)
- Romanian Hemophilia Association
- Ukrainian Federation of Hemophilia
- Swedish Hemophilia Society
Blood and factor safety groups
Additional information
- Heamophilia Life - A resource for patients, carers and healthcare professionals.
- La Kelley Communications - free books and other educational resources
- HemophiliaMoms.com a Resource for Hemophilia Families
- Haemophiliacare.co.uk - A resource for patients and carers
- Simple haemophilia overview for children
- Haemophilia timeline
References
- ↑ Douglas Harper. "Online Etymology Dictionary".
- ↑ 2.0 2.1 "Hemophilia B". Retrieved on 2007-11-21.
- ↑ www.wrongdianosis.com/h/hemophilia/stats.htm#medical_stats
- ↑ World Federation of Hemophilia Frequently Asked Questions About Hemophilia
- ↑ "U.S. National Library of Medicine". Retrieved on 2007-12-02.
- ↑ Canadian Hemophilia Society FAQ
- ↑ Tractate Yevamot 64b
- ↑ Patricia Skinner (2001), Unani-tibbi, Encyclopedia of Alternative Medicine
- ↑ "The History of hemophilia". Retrieved on 2007-06-27.
- ↑ In re Rhone-Poulenc Rorer Inc., 51 F.3d 1293, 1296 (7th Cir. 1995), http://www.projectposner.org/case/1995/51F3d1293 (PDF courtesy link). Retrieved 2008-01-28
- ↑ Manco-Johnson MJ, Abshire TC, Shapiro AD, et al (2007). "Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia". N. Engl. J. Med. 357 (6): 535–44. doi:. PMID 17687129. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=17687129&promo=ONFLNS19.
- ↑ Roosendaal G, Lafeber F (2007). "Prophylactic treatment for prevention of joint disease in hemophilia--cost versus benefit". N. Engl. J. Med. 357 (6): 603–5. doi:. PMID 17687136. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=17687136&promo=ONFLNS19.
- ↑ Mulder, K. 2006. Exercises for People with Hemophilia. World Federation of Hemophilia.
- ↑ 14.0 14.1 University of Maryland Medical Center Complementary medicines: Hemophilia
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||