High-density lipoprotein
High-density lipoproteins (HDL) is one of the 5 major groups of lipoproteins (chylomicrons, VLDL, IDL, LDL, HDL) which enable lipids like cholesterol and triglycerides to be transported within the water based blood stream. In healthy individuals, about thirty percent of blood cholesterol is carried by HDL [1].
It is hypothesized that HDL can remove cholesterol from atheroma within arteries and transport it back to the liver for excretion or re-utilization—which is the main reason why HDL-bound cholesterol is sometimes called "good cholesterol", or HDL-C. A high level of HDL-C seems to protect against cardiovascular diseases, and low HDL cholesterol levels (less than 40 mg/dL) increase the risk for heart disease.[1] When measuring cholesterol, any contained in HDL particles is considered as protection to the body's cardiovascular health, in contrast to "bad" LDL cholesterol.
Contents |
Structure and function
HDL are the smallest of the lipoprotein particles. They are the densest because they contain the highest proportion of protein. Their most abundant apolipoproteins are apo A-I and apo A-II.[2] The liver synthesizes these lipoproteins as complexes of apolipoproteins and phospholipid, which resemble cholesterol-free flattened spherical lipoprotein particles. They are capable of picking up cholesterol, carried internally, from cells by interaction with the ATP Binding Cassette Transporter A1 (ABCA1). A plasma enzyme called lecithin-cholesterol acyltransferase (LCAT) converts the free cholesterol into cholesteryl ester (a more hydrophobic form of cholesterol) which is then sequestered into the core of the lipoprotein particle eventually making the newly synthesized HDL spherical. They increase in size as they circulate through the bloodstream and incorporate more cholesterol and phospholipid molecules from cells and other lipoproteins, for example by the interaction with the ABCG1 transporter and the phospholipid transport protein (PLTP).
HDL deliver their cholesterol mostly to the liver or steroidogenic organs such as adrenals, ovary and testes by direct and indirect pathways. The direct HDL removal pathways involve HDL receptors such as scavenger receptor BI (SR-BI) which mediate the selective uptake of cholesterol from HDL. In humans, the probably most relevant pathway is the indirect one, which is mediated by cholesteryl ester transfer protein (CETP). This protein exchanges triglycerides of VLDL against cholesteryl esters of HDL. As the result, VLDL are processed to LDL which are removed from the circulation by the LDL receptor pathway. The triglycerides are not stable in HDL, but degraded by hepatic lipase so that finally small HDL particles are left which restart the uptake of cholesterol from cells.
The cholesterol delivered to the liver is excreted into the bile and hence intestine either directly or indirectly after conversion into bile acids. Delivery of HDL cholesterol to adrenals, ovaries and testes are important for the synthesis of steroid hormones.
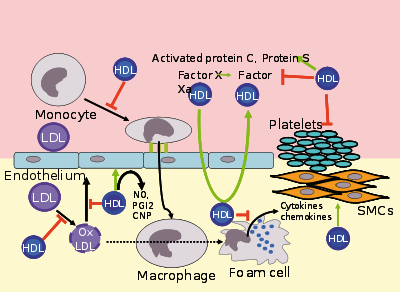
Several steps in the metabolism of HDL can contribute to the transport of cholesterol from lipid laden macrophages of atherosclerotic arteries, termed foam cells to the liver for secretion into the bile. This pathway has been termed reverse cholesterol transport and is considered as the classical protective function of HDL towards atherosclerosis.
However, HDL carries many lipid and protein species, many of which have very low concentrations but are biologically very active. For example, HDL and their protein and lipid constituents help to inhibit oxidation, inflammation, activation of the endothelium, coagulation or platelet aggregation. All these properties may contribute to the ability of HDL to protect from atherosclerosis, and it is not yet known what is most important.
In the stress response, serum amyloid A, which is one of the acute phase proteins and an apolipoprotein, is under the stimulation of cytokines (IL-1, IL-6) and cortisol produced in the adrenal cortex and carried to the damaged tissue incorporated into HDL particles. At the inflammation site, it attracts and activates leukocytes. In chronic inflammations, its deposition in the tissues manifests itself as amyloidosis.
It has been postulated that the concentration of large HDL particles more accurately reflects protective action, as opposed to the concentration of total HDL particles.[3] This ratio of large HDL to total HDL particles varies widely and is only measured by more sophisticated lipoprotein assays using either electrophoresis (the original method developed in the 1970s), or newer NMR spectroscopy methods (See also: NMR and spectroscopy), developed in the 1990s.
Epidemiology
Men tend to have noticeably lower HDL levels, with smaller size and lower cholesterol content, than women. Men also have an increased incidence of atherosclerotic heart disease.
Epidemiological studies have shown that high concentrations of HDL (over 60 mg/dL) have protective value against cardiovascular diseases such as ischemic stroke and myocardial infarction. Low concentrations of HDL (below 40 mg/dL for men, below 50 mg/dL for women) increase the risk for atherosclerotic diseases.
Data from the landmark Framingham Heart Study showed that for a given level of LDL, the risk of heart disease increases 10-fold as the HDL varies from high to low. Conversely, for a fixed level of HDL, the risk increases 3-fold as LDL varies from low to high.
Even people with very low LDL levels are exposed to some increased risk if their HDL levels are not high enough.[4]
Recommended range
The American Heart Association, NIH and NCEP provides a set of guidelines for male fasting HDL levels and risk for heart disease.
| Level mg/dL | Level mmol/L | Interpretation |
| <40 | <1.03 | Low HDL cholesterol, heightened risk for heart disease, <50 is the value for women |
| 40–59 | 1.03–1.52 | Medium HDL level |
| >60 | >1.55 | High HDL level, optimal condition considered protective against heart disease |
Measuring HDL
Many laboratories used a two-step method : chemical precipitation of lipoproteins containing apoprotein B, then calculating HDL as cholesterol remaining in the supernate [5] but there are also direct methods [6]. Labs use the routine dextran sulfate-Mg2+ precipitation method with ultracentrifugation/dextran sulfate-Mg2+ precipitation as reference method [7]. HPLC can be used [8]. See also [9].
Subfractions (HDL-2C, HDL-3C) can be measured [10] and have clinical significance.
Memory
A link has been shown between level of HDL and onset of dementia. Those with high HDL were less likely to have dementia.[11] Low HDL-C in late-middle age has also been associated with memory loss.[12]
Raising HDL
Drugs
Pharmacological therapy to increase the level of HDL cholesterol includes use of fibrates and niacin. Consumption of pharmacologic doses of niacin can increase HDL levels by 10–30%,[13] and it is the most powerful agent currently available to increase HDL-cholesterol.[14][15] A randomized clinical trial demonstrated that such treatments can significantly reduce atherosclerosis progression and cardiovascular events.[16] However, niacin products sold as "no-flush", i.e. not having side effects such as "niacin flush", do not contain free nicotinic acid and are therefore ineffective at raising HDL, while products sold as "sustained release" may contain free nicotinic acid, but "some brands are hepatotoxic"; therefore the recommended form of niacin for raising HDL is the cheapest, immediate release preparation. [17]
In contrast, while the use of statins is effective against high levels of LDL cholesterol, it has little or no effect in raising HDL cholesterol.[14]
Torcetrapib, a promising new drug developed by Pfizer to raise HDL by inhibition of cholesteryl ester transfer protein (CETP), was terminated after a greater percentage of patients treated with torcetrapib-Lipitor combination died compared with patients treated with Lipitor alone. Merck is currently researching a similar molecule called anacetrapib.
Diet and lifestyle
Certain changes in lifestyle can have a positive impact on raising HDL levels:[18]
- Aerobic exercise[19]
- Weight loss
- Smoking cessation
- Removing trans fatty acids from the diet
- One drink of alcohol a day or less yields higher HDL-C levels, more so in women than men. HDL transports cholesterol to the liver and cholesterol is known to have a protective effect on the cell membrane. It is likely that this reflects the liver's need for more cholesterol to protect itself from the alcohol.[20]
- Adding soluble fiber to diet
- Using supplements such as omega 3 fish oil[21]
- Limiting intake of dietary fat to 30–35% of total calories
See also
- Asymmetric dimethylarginine
- Cardiovascular disease
- Endothelium
- Low density lipoprotein
Footnotes
- ↑ 1.0 1.1 LDL and HDL Cholesterol: What's Bad and What's Good?
- ↑ Baylor College of Medicine, Lipids Online (January 29, 2001). "Heterogeneity of HDL". Retrieved on February 20, 2006.
- ↑ Kwiterovich PO. The Metabolic Pathways of High-Density Lipoprotein, Low-Density Lipoprotein, and Triglycerides: A Current Review. Am J Cardiol 2000;86(suppl):5L.
- ↑ Philip Barter, M.D. HDL Cholesterol, Very Low Levels of LDL Cholesterol, and Cardiovascular Events, September 27, 2007; NEJM
- ↑ http://www.clinchem.org/cgi/content/short/44/5/1050
- ↑ http://www.med.umich.edu/mdrtc/cores/ChemCore/lipids.htm Lipid Measurement Fact Sheet
- ↑ http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TDD-3X5GP14-5&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=ee1cd62ecd1496a88a9e46b8e4ff1b90 Homogeneous HDL-cholesterol assay versus ultracentrifugation/dextran sulfate-Mg2+ precipitation and dextran sulfate-Mg2+ precipitation. 1998
- ↑ http://www.citeulike.org/user/biblio24/article/2461070 Evaluation of precipitation and direct methods for HDL-cholesterol assay by HPLC. 1997
- ↑ Clinical Chemistry By Larry E. Schoeff
- ↑ http://www.jlr.org/cgi/content/abstract/49/5/1130 measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay
- ↑ New York Times July 1st 2008
- ↑ Low HDL cholesterol is a risk factor for deficit a...[Arterioscler Thromb Vasc Biol. 2008] - PubMed Result
- ↑ "Raising HDL in Clinical Practice". Retrieved on December 16, 2007.
- ↑ 14.0 14.1 Raising HDL-Cholesterol and Reducing Cardiovascular Risk. Medscape Cardiology http://www.medscape.com/viewarticle/520393
- ↑ Chapman M, Assmann G, Fruchart J, Shepherd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid - a position paper developed by the European Consensus Panel on HDL-C. Cur Med Res Opin. 2004 Aug;20(8):1253-68. PubMed
- ↑ Reducing risk by raising HDL-cholesterol: the evidence. # European Heart Journal Supplements Vol 8 Suppl F p. F23-F29 http://eurheartjsupp.oxfordjournals.org/cgi/content/abstract/8/suppl_F/F23
- ↑ "Varying Cost and Free Nicotinic Acid Content in Over-the-Counter Niacin Preparations for Dyslipidemia"; C. Daniel Meyers, MD; Molly C. Carr, MD; Sang Park, PhD; and John D. Brunzell, MD; Annals of Internal Medicine 139, issue 12; December 16, 2003, pages 996-1002
- ↑ Richard N. Fogoros, M.D.. "Raising Your HDL Levels". Retrieved on July 29, 2006.
- ↑ Spate-Douglas, T., Keyser, R. E. Exercise intensity: its effect on the high-density lipoprotein profile. Arch Phys Med Rehabil 80, 691-695. PubMed
- ↑ Gerdi Weidner, PhD; Sonja L. Connor, MS, RD; Margaret A. Chesney, PhD; John W. Burns, MA; William E. Connor, MD; Joseph D. Matarazzo, PhD; and Nancy R. Mendell, PhD. "Sex, alcohol, and HDL - high-density lipoprotein cholesterol; Family Heart Study, Portland, Oregon" (PDF). Retrieved on March 14, 2008.
- ↑ "The Power of Fish". The Cleveland Clinic Heart and Vascular Institute. Retrieved on July 21, 2008.
External links
|
|||||||||||||||||