Glutamic acid
| Glutamic acid | |
|---|---|
 |
 |
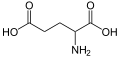
| IUPAC name | (2S)-2-aminopentanedioic acid |
| Identifiers | |
| CAS number | 56-86-0 |
| PubChem | |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C5H9NO4 |
| Molar mass | 147.13 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Glutamic acid (abbreviated as Glu or E) is one of the 20 proteinogenic amino acids and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates.
Contents |
Chemistry
The side chain carboxylic acid functional group has pKa of 4.1 and exists in its negatively charged deprotonated carboxylate form at physiological pH.
History
This compound was discovered in 1908 by the professor Kikunae Ikeda, who worked in the Imperial University of Tokyo. He loved seaweed, which is used like spice in traditional Japanese food. He tried to find the root of this flavour. He discovered that the origin of this taste is glutamic acid. He isolated crystals of glutamic acid using a Kombu soup (one hundred grams of Kombu has nearly one gram of glutamic acid).
Moreover, he discovered that glutamate gave unique flavour to other foods. He called it "Umami" (meaning "yumminess" in Japanese). This distinctive flavor has brought glutamate the title of "the elusive fifth taste" to join the more traditional flavors, sweet, salty, sour, and bitter. [1]
Biosynthesis
| Reactants | Products | Enzymes |
|---|---|---|
| Glutamine + H2O | → Glu + NH3 | GLS, GLS2 |
| NAcGlu + H2O | → Glu + Acetate | (unknown) |
| α-ketoglutarate + NADPH + NH4+ | → Glu + NADP+ + H2O | GLUD1, GLUD2 |
| α-ketoglutarate + α-amino acid | → Glu + α-oxo acid | transaminase |
| 1-pyrroline-5-carboxylate + NAD+ + H2O | → Glu + NADH | ALDH4A1 |
| N-formimino-L-glutamate + FH4 | → Glu + 5-formimino-FH4 | FTCD |
Function and uses
Metabolism
Glutamate is a key molecule in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serves as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase. The reaction can be generalised as such:
- R1-amino acid + R2-α-ketoacid ⇌ R1-α-ketoacid + R2-amino acid
A very common α-ketoacid is α-ketoglutarate, an intermediate in the citric acid cycle. Transamination of α-ketoglutarate gives glutamate. The resulting α-ketoacid product is often a useful one as well, which can contribute as fuel or as a substrate for further metabolism processes. Examples are as follows:
- Aspartate + α-ketoglutarate ⇌ oxaloacetate + glutamate
Both pyruvate and oxaloacetate are key components of cellular metabolism, contributing as substrates or intermediates in fundamental processes such as glycolysis, gluconeogenesis and also the citric acid cycle.
Glutamate also plays an important role in the body's disposal of excess or waste nitrogen. Glutamate undergoes deamination, an oxidative reaction catalysed by glutamate dehydrogenase, as follows:
- glutamate + water + NADP+ → α-ketoglutarate + NADPH + ammonia + H+
Ammonia (as ammonium) is then excreted predominantly as urea, synthesised in the liver. Transamination can thus be linked to deamination, effectively allowing nitrogen from the amine groups of amino acids to be removed, via glutamate as an intermediate, and finally excreted from the body in the form of urea.
Neurotransmitter
Glutamate is the most abundant excitatory neurotransmitter in the mammalian nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, glutamate receptors, such as the NMDA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, it is believed that glutamic acid is involved in cognitive functions like learning and memory in the brain.
Glutamate transporters[3] are found in neuronal and glial membranes. They rapidly remove glutamate from the extracellular space. In brain injury or disease, they can work in reverse and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via NMDA receptor channels, leading to neuronal damage and eventual cell death, and is called excitotoxicity. The mechanisms of cell death include
- Damage to mitochondria from excessively high intracellular Ca2+;[4]
- Glu/Ca2+-mediated promotion of transcription factors for pro-apoptotic genes, or downregulation of transcription factors for anti-apoptotic genes.
Excitotoxicity due to glutamate occurs as part of the ischemic cascade and is associated with stroke and diseases like amyotrophic lateral sclerosis, lathyrism, autism, some forms of mental retardation and Alzheimer's disease.
Glutamic acid has been implicated in epileptic seizures. Microinjection of glutamic acid into neurons produces spontaneous depolarisations around one second apart, and this firing pattern is similar to what is known as paroxysmal depolarizing shift in epileptic attacks. This change in the resting membrane potential at seizure foci could cause spontaneous opening of voltage-activated calcium channels, leading to glutamic acid release and further depolarization.
Experimental techniques to detect glutamate in intact cells include using a genetically-engineered nanosensor[2]. The sensor is a fusion of a glutamate-binding protein and two fluorescent proteins. When glutamate binds, the fluorescence of the sensor under ultraviolet light changes by resonance between the two fluorophores. Introduction of the nanosensor into cells enables optical detection of the glutamate concentration. Synthetic analogs of glutamic acid that can be activated by ultraviolet light have also been described[6]. This method of rapidly uncaging by photostimulation is useful for mapping the connections between neurons, and understanding synapse function.
Brain nonsynaptic glutamatergic signaling circuits
Extracellular glutamate in Drosophilia brains has been found to regulate postsynaptic glutamate receptor clustering, via a process involving receptor desensitization[7]. A gene expressed in glial cells actively transports glutamate into the extracellular space[7], while in the nucleus accumbens stimulating group II metabotropic glutamate receptors, this gene was found to reduce extracellular glutamate levels[8]. This raises the possibility that this extracellular glutamate plays an "endocrine-like" role as part of a larger homeostatic system.
GABA precursor
Glutamic acid also serves as the precursor for the synthesis of the inhibitory GABA in GABA-ergic neurons. This reaction is catalyzed by glutamic acid decarboxylase (GAD), which is most abundant in the cerebellum and pancreas.
Stiff-man syndrome is a neurologic disorder caused by anti-GAD antibodies, leading to a decrease in GABA synthesis and therefore, impaired motor function such as muscle stiffness and spasm. Since the pancreas is also abundant for the enzyme GAD, a direct immunological destruction occurs in the pancreas and the patients will have diabetes mellitus.
Flavor enhancer
Free glutamic acid is present in a wide variety of foods, including soy sauce and is responsible for one of the five basic tastes of the human sense of taste (umami). Glutamic acid is often used as a food additive and flavour enhancer in the form of its sodium salt, monosodium glutamate (MSG).
Nutrient
All meats, poultry, fish, eggs, dairy products, as well kombu are excellent sources of glutamic acid. Some protein-rich plant foods also serve as sources. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass [5]
Plant growth
Auxigro is a plant growth preparation that contains 30% glutamic acid.
Production
China-based Fufeng Group Limited is the largest producer of glutamic acid in the world, with capacity increasing to 300,000 tons at the end of 2006 from 180,000 tons during 2006, putting them at 25%–30% of the Chinese market. Meihua is the second largest Chinese producer. Together, the top five producers have roughly 50% share in China. Chinese demand is roughly 1.1 million tons per year, while global demand, including China, is 1.7 million tons per year.
Pharmacology
The drug phencyclidine (more commonly known as PCP) antagonizes glutamic acid non-competitively at the NMDA receptor. For the same reasons, sub-anaesthetic doses of Ketamine have strong dissociative and hallucinogenic effects. Glutamate does not easily pass the blood brain barrier, but instead this transport is mediated by a high affinity transport system.[1] It can also be converted into glutamine.
Role in sickle-cell disease
A point mutation (valine in place of glutamic acid at position 6) in the β-globin chain of hemoglobin forms HbS. This variant of hemoglobin can cause sickle-cell anemia, where the abnormal hemoglobin are prone to polymerization when deoxygenated, thus distorting the erythrocyte which are removed by the spleen or cause microvascular obstruction (ischemic crises). This trait and disease is common in areas with a high prevalence of Plasmodium falciparum (one of three Plasmodium species that causes malaria).
See also
- Kainic acid
References
Other
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry, 4th edition.
- a
 Okumoto, S., et al. (2005). "Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors". Proceedings of the National Academy of Sciences U.S.A 102 (24): 8740–8745. doi:. PMID 15939876. Free text
Okumoto, S., et al. (2005). "Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors". Proceedings of the National Academy of Sciences U.S.A 102 (24): 8740–8745. doi:. PMID 15939876. Free text - a Shigeri Y, Seal RP, Shimamoto K (July 2004). "Molecular pharmacology of glutamate transporters, EAATs and VGLUTs". Brain Res. Brain Res. Rev. 45 (3): 250–65. doi:. PMID 15210307.
- a Manev H, Favaron M, Guidotti A, Costa E (July 1989). "Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death". Mol. Pharmacol. 36 (1): 106–12. PMID 2568579. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=2568579.
- a
 Reeds, P.J., et al. (2000). "Intestinal glutamate metabolism". Journal of Nutrition 130 (4s): 978S–982S. PMID 10736365. Free text
Reeds, P.J., et al. (2000). "Intestinal glutamate metabolism". Journal of Nutrition 130 (4s): 978S–982S. PMID 10736365. Free text -
 Corrie, J.E., et al. (1993). "Postsynaptic activation at the squid giant synapse by photolytic release of L-glutamate from a 'caged' L-glutamate". Journal of Physiology 465 (Jun): 1–8. PMID 7901400. Free text
Corrie, J.E., et al. (1993). "Postsynaptic activation at the squid giant synapse by photolytic release of L-glutamate from a 'caged' L-glutamate". Journal of Physiology 465 (Jun): 1–8. PMID 7901400. Free text - Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE (2007). "Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo". Journal of Neuroscience 27 (1): 111–123. doi:. PMID 17202478.
- Zheng Xi, Baker DA, Shen H, Carson DS, Kalivas PW (2002). "Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens". Journal of Pharmacology and Experimental Therapeutics 300 (1): 162–171. doi:. PMID 11752112.
|
||||||||||||||
|
||||||||||||||||||||||||