Fluoxetine
 |
|
 |
|
|
Fluoxetine
|
|
| Systematic (IUPAC) name | |
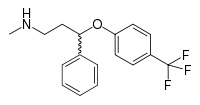
| N-methyl-3-phenyl-3-[4- (trifluoromethyl)phenoxy]propan-1-amine | |
| Identifiers | |
| CAS number | |
| ATC code | N06 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C17H18F3NO |
| Mol. mass | 309.3 g/mol (345.8 for •HCl) |
| Pharmacokinetic data | |
| Bioavailability | 72% peak at 6-8 hours |
| Protein binding | 94.5% |
| Metabolism | Hepatic |
| Half life | 1-3 days (acute); 4-6 days (chronic); Active metabolite Norfluoxetine 4-16 days (acute and chronic) |
| Excretion | Kidneys 80%, intestines 15% |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. | |
| Legal status |
℞ Prescription only |
| Routes | Oral |
Fluoxetine hydrochloride (trade names Prozac, Fontex, Ladose, Sarafem) is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Fluoxetine is approved for the treatment of major depression (including pediatric depression), obsessive-compulsive disorder (in both adult and pediatric populations), bulimia nervosa, anorexia nervosa, panic disorder and premenstrual dysphoric disorder.[1] Despite the availability of newer agents, it remains extremely popular. Over 22.2 million prescriptions for generic formulations of fluoxetine were filled in the United States in 2007, making it the third most prescribed antidepressant.[2] Fluoxetine was developed by Eli Lilly and Company, by scientists Klaus Schmiegel and Bryan Molloy. Lilly also markets the drug for use with pets under the Reconcile brand name.
Contents |
History
According to David Wong,[3] the work which eventually led to the discovery of fluoxetine began at Eli Lilly in 1970 as a collaboration between Bryan Molloy and Robert Rathbun. It was known at that time that the antihistamine diphenhydramine shows some antidepressant-like properties. 3-Phenoxy-3-phenylpropylamine, a compound structurally similar to diphenhydramine, was taken as a starting point, and Molloy synthesized dozens of its derivatives. Testing the physiological effects of these compounds in mice resulted in nisoxetine, a selective norepinephrine reuptake inhibitor currently widely used in biochemical experiments.[3]
Later, hoping to find a derivative inhibiting only serotonin reuptake, Wong proposed to re-test the series for the in-vitro reuptake of serotonin, norepinephrine and dopamine. This test, carried out by Jong-Sir Horng in May 1972,[3] showed the compound later named fluoxetine to be the most potent and selective inhibitor of serotonin reuptake of the series.[4]
A controversy ensued after Lilly researchers published a paper entitled "Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug"[3] implicitly claiming fluoxetine to be the first selective serotonin reuptake inhibitor (SSRI). Two years later they had to issue a correction, admitting that the first SSRI was zimelidine developed by Arvid Carlsson and colleagues.[5] Fluoxetine made its appearance on the Belgian market in 1986[6] and was approved for use by the FDA in the United States in December 1987.[7] Fluoxetine was the fourth SSRI to make it to market, after zimelidine, indalpine and fluvoxamine. However, the first two were withdrawn due to the side effects, and a vigorous marketing campaign by Eli Lilly made sure that in the popular culture fluoxetine has been perceived as a scientific breakthrough and associated with the title of the first SSRI.
Eli Lilly's patent on Prozac (fluoxetine) expired in August, 2001,[8] prompting an influx of generic drugs onto the market.
Indications
Fluoxetine has been approved by the FDA for the treatment of major depression, obsessive compulsive disorder, bulimia nervosa and panic disorder.[9] Fluoxetine was shown to be effective for depression in 6-week long double-blind controlled trials where it also alleviated anxiety and improved sleep. Fluoxetine was better than placebo for the prevention of depression recurrence when the patients, who originally responded to fluoxetine, were treated for a further 38 weeks. Efficacy of fluoxetine for geriatric as well as pediatric depression was also demonstrated in placebo-controlled trials.[9]
The peculiar pharmacokinetics of fluoxetine with its brain levels rising extremely slowly over at least first 5 weeks of treatment (see #Pharmacokinetics) makes it unclear whether the 20-mg/day optimal dose established in the short term (6-8 weeks) trials is applicable for the longer term supportive treatment. One 60-mg dose of fluoxetine per week was found to be equivalent to 20 mg/day for the continuation treatment of responders to 20 mg/day of fluoxetine.[10][11] Furthermore, 5 mg/day fluoxetine was shown to be better than placebo and similar to 20 mg/day,[12] and one weekly dose of 80 mg fluoxetine was equivalent to 60 mg/day fluoxetine or 150 mg/day amitriptyline.[11] On the other hand, increase of the dose to 60 mg/day in non-responders from 20 mg/day brought no additional benefits as compared to continuing the 20 mg/day treatment.[12]
The recent research suggests that a significant part of the resistance to the SSRIs paroxetine (Paxil) and citalopram (Celexa) can be explained by the genetic variation of Pgp transporter. Paroxetine and citalopram, which are Pgp substrates, are actively transported from the brain by this protein. Fluoxetine is not a substrate of Pgp, and thus a switch from paroxetine or citalopram to fluoxetine may be beneficial to the non-responders.[13][14]
OCD was successfully treated by fluoxetine in two adult and one pediatric placebo-controlled 13-week trials. The higher doses of fluoxetine appeared to result in better response, while the reverse relationship was observed in the treatment of depression.[9] Fluoxetine dramatically, by 40-50%, decreased the frequency of panic attacks in two controlled trials of panic disorder patients. In three double-blind trials fluoxetine significantly decreased the number of binge-eating and purging episodes of bulimia nervosa. Continued year-long treatment of the patients, who originally responded to fluoxetine, was more effective than placebo for the prevention of bulimia nervosa episodes.[9]
Pharmacokinetics

The bioavailability of fluoxetine is relatively high (72%), and peak plasma concentrations are reached in 6 to 8 hours. It is highly bound to plasma proteins, mostly albumin.
Fluoxetine is metabolized in the liver by isoenzymes of the cytochrome P450 system, including CYP2D6.[1] The role of CYP2D6 in the metabolism of fluoxetine may be clinically important, as there is great genetic variability in the function of this enzyme among people. Only one metabolite of fluoxetine, norfluoxetine (demethylated fluoxetine), is biologically active.
The extremely slow elimination of fluoxetine and its active metabolite norfluoxetine from the body distinguishes it from other antidepressants. With time, fluoxetine and norfluoxetine inhibit their own metabolism, so fluoxetine elimination half-life changes from 1 to 3 days, after a single dose—to 4 to 6 days, after long-term use. Similarly, the half-life of norfluoxetine is longer (16 days) after long-term use.[1][17][11] Therefore, the concentration of the drug and its active metabolite in the blood continues to grow through the first few weeks of treatment, and their steady concentration in the blood is achieved only after four weeks.[18][19] Moreover, the brain concentration of fluoxetine and its metabolites keeps increasing through at least the first five weeks of treatment.[20] That means that the full benefits of the current dose a patient receives are not realized for at least a month since its initiation. For example, in one 6-week study, the median time to achieving consistent response was 29 days.[18] Likewise, complete excretion of the drug may take several weeks. During the first week after the treatment discontinuation, the brain concentration of fluoxetine decreases only by 50%,[20] The blood level of norfluoxetine 4 weeks after the treatment discontinuation is about 80% of the level registered by the end of the first treatment week, and 7 weeks after the discontinuation norfluoxetine is still detectable in the blood.[11]
A PET study compared the action of a single dose of fluoxetine on exclusively heterosexual and exclusively homosexual men who attested that their past and present sexual behavior, desires, and fantasies were directed entirely toward women or men, respectively. The study found that in some areas of the brain the metabolic response in these two groups was different. "Both groups, however, did exhibit similar widespread lateralized metabolic responses to fluoxetine (relative to placebo), with most areas of the brain responding in the same direction." They "did not differ on behavioral measures or blood levels of fluoxetine".[21]
Adverse effects

According to the manufacturer of Prozac brand of fluoxetine Eli Lilly, fluoxetine is contraindicated in individuals taking monoamine oxidase inhibitors, pimozide (Orap) or thioridazine (Mellaril).[9] The prescribing information recommends that the treatment of the patients with liver impairment "must be approached with caution". The elimination of fluoxetine and its metabolite norfluoxetine is about twice slower in these patients, resulting in the proportionate increase of exposure to the drug.[9]
Among the common adverse effects associated with fluoxetine and listed in the prescribing information, the effects with the greatest difference from placebo are nausea (22% vs 9% for placebo), insomnia (19% vs 10% for placebo), somnolence (12% vs 5% for placebo), anorexia (10% vs 3% for placebo), anxiety (12% vs 6% for placebo), nervousness (13% vs 8% for placebo), asthenia (11% vs 6% for placebo) and tremor (9% vs 2% for placebo). Those that most often resulted in interruption of the treatment were anxiety, insomnia, and nervousness (1-2% each), and in pediatric trials—mania (2%).[9]
In addition, rash or urticaria, sometimes serious, was observed in 7% patients in clinical trials; one-third of these cases resulted in discontinuation of the treatment. Postmarketing reports note several cases of complications developed in patients with rash. The symptoms included vasculitis and lupus-like syndrome. Death has been reported to occur in association with these systemic events.[9]
Akathisia, that is inner tension, restlessness, and the inability to stay still, often accompanied by "constant pacing, purposeless movements of the feet and legs, and marked anxiety," is a common side effect of fluoxetine.[22][23] Akathisia usually begins after the initiation of the treatment or increase of the dose and disappears after fluoxetine is stopped or its dose is decreased, or after treatment with propranolol.[24][25][22] There are case reports directly linking akathisia with suicidal attempts, with patients feeling better after the withdrawal of fluoxetine, and again developing severe akathisia on repeated exposure to fluoxetine. These patients described "that the development of the akathisia made them feel suicidal and that it had precipitated their prior suicide attempts."[25] The experts note that because of the link of akathisia with suicide and the distress it causes to the patient, "it is of vital importance to increase awareness amongst staff and patients of the symptoms of this relatively common condition".[26][27] More rarely, fluoxetine has been associated with related movement disorders acute dystonia and tardive dyskinesia.[23][28][29]
Other side effects may occur, including sexual dysfunction. Possible sexual side effects can include anorgasmia, reduced libido and impotence.[30]
Fluoxetine taken during pregnancy also increases rate of poor neonatal adaptation.[30] Because fluoxetine is excreted in human milk, nursing while on fluoxetine is not recommended.[31] The American Association of Pediatrics classifies fluoxetine as a drug for which the effect on the nursing infant is unknown but may be of concern.[32]
Discontinuation syndrome
Several case reports in the literature describe severe withdrawal or discontinuation symptoms following an abrupt interruption of fluoxetine treatment.[33] Considering the number of fluoxetine prescriptions dispensed over the years, this is exceedingly rare. It is generally believed that the side effects of the fluoxetine discontinuation are mild,[33] and one of the recommended strategies for the management of discontinuation syndrome with other SSRIs is to substitute fluoxetine for the original agent.[34][35] The double-blind controlled studies support this opinion. No increase in side effects was observed in several studies when the treatment with fluoxetine was blindly interrupted for a short time (4-8 days) and then re-instated, this result being consistent with its slow elimination from the body. More side effects occurred during the interruption of sertraline in these studies, and significantly more—during the interruption of paroxetine.[36] In a longer, 6 week-long, blind discontinuation study, insignificantly higher (32% vs 27%) overall rate of new or worsened side effects was observed in the group that discontinued fluoxetine than in the group that continued treatment. However, significantly higher 4% rate of somnolence at week 2 and 5-7% rate of dizziness at weeks 4-6 were reported by the patients in the discontinuation group. This prolonged course of the discontinuation symptoms, with dizziness persisting to the end of the study, is also consistent with the long half-life of fluoxetine in the body.[37]
Suicidality in antidepressant trials
The FDA requires all antidepressants, including fluoxetine, to carry a black box warning stating that antidepressants may increase the risk of suicide in persons younger than 25. This warning is based on statistical analyses conducted by two independent groups of the FDA experts that found a 2-fold increase of the suicidal ideation and behavior in children and adolescents, and 1.5-fold increase of suicidality in the 18–24 age group. The suicidality was slightly decreased for those older than 24, and statistically significantly lower in the 65 and older group.[38][39][40] This analysis was criticized by Donald Klein who noted that suicidality, that is suicidal ideation and behavior, is not necessarily a good surrogate marker for completed suicide, and it is still possible that antidepressants may prevent actual suicide while increasing suicidality.[41] This opinion goes against the general consensus that "suicidal ideation has been associated with suicide attempt in retrospective studies and with suicide in prospective studies."[42]
Suicidality and fluoxetine
Suicidal ideation and behavior in clinical trials are rare. For the above analysis the FDA combined the results of 295 trials of 11 antidepressants for psychiatric indications in order to obtain statistically significant results. Considered separately, fluoxetine use in children increased the odds of suicidality by 50% (not statistically significant),[43] and in adults decreased the odds of suicidality by approximately 30% (statistically significant).[39][40] Similarly, the analysis conducted by the UK MHRA found a 50% increase of odds of suicide-related events, not reaching statistical significance, in the children and adolescents on fluoxetine as compared to the ones on placebo. According to the MHRA data, for adults fluoxetine did not change the rate of self-harm and statistically significantly decreased suicidal ideation by 50%.[44][45]
Interactions
The simultaneous use of fluoxetine with triptans, tramadol or other serotonergic agents can result in a rare, but potentially life-threatening adverse drug reaction called serotonin syndrome.
Controversy
"In 1989, Joseph Wesbecker shot dead eight people and injured 12 others before killing himself at his place of work in Kentucky. Wesbecker had been taking the selective serotonin reuptake inhibitor (SSRI) antidepressant fluoxetine for four weeks before these homicides, and this led to a legal action against the makers of fluoxetine, Eli Lilly.[46] The case was tried and settled in 1994, and as part of the settlement a number of pharmaceutical company documents about drug-induced activation were released into the public domain. Subsequent legal cases...have further raised the possibility of a link between antidepressant use and violence."[47]
A meta-analysis published in February 2008 combined 35 clinical trials of four newer antidepressants (fluoxetine, paroxetine (Paxil), nefazodone (Serzone) and venlafaxine (Effexor)). These antidepressants belonging to three different pharmacological groups were considered together, and the authors did not analyze them separately. The authors concluded that "although the difference [between the placebo and antidepressants] easily attained statistical significance", it did not meet the criterion for clinical significance, as used by National Institute for Health and Clinical Excellence (UK), "for any but the most severely depressed patients."[48] Some articles in the press using the titles "The creation of the Prozac myth"[49] and "Prozac does not work in majority of depressed patients"[50][51] presented these general findings about the relative efficacy of antidepressants and placebo as the findings about ineffectiveness of fluoxetine.
Prozac in popular culture
Because of its wide appeal as a popular anti-depressant, Prozac has had numerous references to it in popular culture, including many books, movies, and songs. For example, the autobiographical book Prozac Nation was written in 1994 by Elizabeth Wurtzel; it was turned into a movie of the same name, Prozac Nation, in 2001, starring Christina Ricci.
At least three contemporary music groups have derived their name from Prozac, including the punk rock band Prozac+, the rapper Prozak and the pop band Prozzäk.
References
- ↑ 1.0 1.1 1.2 "Prozac Pharmacology, Pharmacokinetics, Studies, Metabolism". RxList.com (2007). Retrieved on 2007-04-14.
- ↑ After sertraline and escitalopram, see: "Top 200 Generic Drugs by Units in 2007". and Verispan (2008-02-18). "Top 200 Brand Drugs by Units in 2007" (PDF). Drug Topics. Retrieved on 2008-03-30.
- ↑ 3.0 3.1 3.2 3.3 Wong, DT, Bymaster FP, Engleman EA (1995). "Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication". Life Sci 57 (5): 411–41. doi:. PMID 7623609.
- ↑ Wong D, Horng J, Bymaster F, Hauser K, Molloy B (1974). "A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine". Life Sci 15 (3): 471–9. doi:. PMID 4549929.
- ↑ Carlsson A, Wong DT (1997). "A note on the discovery of selective serotonin reuptake inhibitors". Life Sci 61 (12): 1203. doi:. PMID 9315511.
- ↑ Swiatek, Jeff (August 2, 2001), "Prozac's profitable run coming to an end for Lilly", The Indianapolis Star, http://www2.indystar.com/library/factfiles/business/companies/lilly/stories/2001_0802.html
- ↑ "Electronic Orange Book". Food and Drug Administration (April 2007). Retrieved on May 24, 2007.
- ↑ "Patent Expiration Dates for Common Brand-Name Drugs". Retrieved on 2007-07-20.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 "Prozac prescribing information" (PDF). Eli Lilly (2007-06-21). Retrieved on 2008-01-09.
- ↑ Burke WJ, Hendricks SE, McArthur-Campbell D, Jacques D, Stull T (1996). "Fluoxetine and norfluoxetine serum concentrations and clinical response in weekly versus daily dosing". Psychopharmacol Bull 32 (1): 27–32. PMID 8927671.
- ↑ 11.0 11.1 11.2 11.3 Burke WJ, Hendricks SE, McArthur-Miller D, Jacques D, Bessette D, McKillup T, Stull T, Wilson J (2000). "Weekly dosing of fluoxetine for the continuation phase of treatment of major depression: results of a placebo-controlled, randomized clinical trial". J Clin Psychopharmacol 20 (4): 423–7. PMID 10917403.
- ↑ 12.0 12.1 Gram L (1994). "Fluoxetine". N. Engl. J. Med. 331 (20): 1354–61. PMID 7935707.
- ↑ Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, Dose T, Ebinger M, Rosenhagen M, Kohli M, Kloiber S, Salyakina D, Bettecken T, Specht M, Pütz B, Binder EB, Müller-Myhsok B, Holsboer F (2008). "Polymorphisms in the Drug Transporter Gene ABCB1 Predict Antidepressant Treatment Response in Depression". Neuron 57 (2): 203–9. doi:. PMID 18215618.
- ↑ Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, Hosoi Y, Takekita Y, Mandelli L, Azuma J, Kinoshita T (2008). "ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder". Prog. Neuropsychopharmacol. Biol. Psychiatry 32 (2): 398–404. doi:. PMID 17913323.
- ↑ Mandrioli R, Forti GC, Raggi MA (February 2006). "Fluoxetine metabolism and pharmacological interactions: the role of cytochrome p450". Current Drug Metabolism 7 (2): 127–33. PMID 16472103. http://www.bentham-direct.org/pages/content.php?CDM/2006/00000007/00000002/0002F.SGM.
- ↑ Hiemke C, Härtter S (January 2000). "Pharmacokinetics of selective serotonin reuptake inhibitors". Pharmacology & Therapeutics 85 (1): 11–28. PMID 10674711. http://linkinghub.elsevier.com/retrieve/pii/S0163-7258(99)00048-0.
- ↑ "Drug Treatments in Psychiatry: Antidepressants". Newcastle University School of Neurology, Neurobiology and Psychiatry (2005). Retrieved on 2007-04-14.
- ↑ 18.0 18.1 Pérez V, Puiigdemont D, Gilaberte I, Alvarez E, Artigas F (2001). "Augmentation of fluoxetine's antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors". J Clin Psychopharmacol 21 (1): 36–45. PMID 11199945.
- ↑ Brunswick DJ, Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Beasley CM (2002). "Fluoxetine and norfluoxetine plasma concentrations during relapse-prevention treatment". J Affect Disord 68 (2-3): 243–9. PMID 12063152.
- ↑ 20.0 20.1 Henry ME, Schmidt ME, Hennen J, Villafuerte RA, Butman ML, Tran P, Kerner LT, Cohen B, Renshaw PF (2005). "A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: A 19-F MRS study". Neuropsychopharmacology 30 (8): 1576–83. doi:. PMID 15886723.
- ↑ Kinnunen LH, Moltz H, Metz J, Cooper M (2004). "Differential brain activation in exclusively homosexual and heterosexual men produced by the selective serotonin reuptake inhibitor, fluoxetine". Brain Res. 1024 (1-2): 251–4. doi:. PMID 15451388.
- ↑ 22.0 22.1 Lipinski JF, Mallya G, Zimmerman P, Pope HG (1989). "Fluoxetine-induced akathisia: clinical and theoretical implications". J Clin Psychiatry 50 (9): 339–42. PMID 2549018.
- ↑ 23.0 23.1 Review:Leo RJ (1996). "Movement disorders associated with the serotonin selective reuptake inhibitors". The Journal of clinical psychiatry 57 (10): 449–54. PMID 8909330.
- ↑ Hansen L (2003). "Fluoxetine dose-increment related akathisia in depression: implications for clinical care, recognition and management of selective serotonin reuptake inhibitor-induced akathisia". J. Psychopharmacol. (Oxford) 17 (4): 451–2. PMID 14870959.
- ↑ 25.0 25.1 Rothschild AJ, Locke CA (1991). "Reexposure to fluoxetine after serious suicide attempts by three patients: the role of akathisia". J Clin Psychiatry 52 (12): 491–3. PMID 1752848.
- ↑ Hansen L (2001). "A critical review of akathisia, and its possible association with suicidal behaviour". Human Psychopharmacology Clinical and Experimental 16 (7): 495–505. doi:. PMID 12404546.
- ↑ Hansen L, Kingdom D (2006). "Akathisia as a risk factor for suicide". The British journal of psychiatry: the journal of mental science 188: 192. doi:. PMID 16449715.
- ↑ Gerber PE, Lynd LD (1998). "Selective serotonin-reuptake inhibitor-induced movement disorders". Ann Pharmacother 32 (6): 692–8. PMID 9640489.
- ↑ Caley CF (1997). "Extrapyramidal reactions and the selective serotonin-reuptake inhibitors". Ann Pharmacother 31 (12): 1481–9. PMID 9416386.
- ↑ 30.0 30.1 NTP_CERHR Expert Panel Report on Reproductive and Developmental Toxicity of fluoxetine, Center for the Evaluation of Risks to Human Reproduction, April 2004, http://cerhr.niehs.nih.gov/chemicals/fluoxetine/fluoxetine_final.pdf
- ↑ Prozac Medication Insert. Eli Lilly and Company Indianapolis, IN 46285, USA Literature revised December 4, 2006
- ↑ "Fluoxetine Drug Information Provided by Lexi-Comp". Merck Manual (June 2007).
- ↑ 33.0 33.1 The most recent case report, see Blum D, Maldonado J, Meyer E, Lansberg M (2008). "Delirium following abrupt discontinuation of fluoxetine". Clin Neurol Neurosurg 110 (1): 69–70. doi:. PMID 17913343.For the earlier case reports see the references cited therein.
- ↑ Rosenbaum JF, Zajecka J (1997). "Clinical management of antidepressant discontinuation". J Clin Psychiatry 58 Suppl 7: 37–40. PMID 9219493.
- ↑ Schatzberg AF, Blier P, Delgado PL, Fava M, Haddad PM, Shelton RC (2006). "Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research". J Clin Psychiatry 67 Suppl 4: 27–30. PMID 16683860.
- ↑ Fava M (2006). "Prospective studies of adverse events related to antidepressant discontinuation". J Clin Psychiatry 67 Suppl 4: 14–21. PMID 16683858.
- ↑ Zajecka J, Fawcett J, Amsterdam J, Quitkin F, Reimherr F, Rosenbaum J, Michelson D, Beasley C (1998). "Safety of abrupt discontinuation of fluoxetine: a randomized, placebo-controlled study". J Clin Psychopharmacol 18 (3): 193–7. PMID 9617977.
- ↑ Levenson M, Holland C. "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". Retrieved on 2007-05-13.
- ↑ 39.0 39.1 Stone MB, Jones ML (2006-11-17). "Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC) 11-74. FDA. Retrieved on 2007-09-22.
- ↑ 40.0 40.1 Levenson M, Holland C (2006-11-17). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC) 75-140. FDA. Retrieved on 2007-09-22.
- ↑ Klein DF (2006). "The flawed basis for FDA post-marketing safety decisions: the example of anti-depressants and children". Neuropsychopharmacology 31 (4): 689–99. doi:. PMID 16395296.
- ↑ Mann JJ, Ellis SP, Waternaux CM, Liu X, Oquendo MA, Malone KM, Brodsky BS, Haas GL, Currier D (2008). "Classification Trees Distinguish Suicide Attempters in Major Psychiatric Disorders: A Model of Clinical Decision Making". J Clin Psychiatry: e1–e9. PMID 18190232.
- ↑ Tarek A. Hammad (2004-09-13). "Results of the Analysis of Suicidality in Pediatric Trials of Newer Antidepressants" (PDF). Presentation at the Meeting of Psychopharmacologic Drugs Advisory Committee and the Pediatric Advisory Committee on September 13, 2004 25, 28. FDA. Retrieved on 2008-01-06.
- ↑ Committee on Safety of Medicines Expert Working Group (December 2004). "Report on The Safety of Selective Serotonin Reuptake Inhibitor Antidepressants" (PDF). MHRA. Retrieved on 2007-09-25.
- ↑ Gunnell D, Saperia J, Ashby D (2005). "Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review". BMJ 330 (7488): 385. doi:. PMID 15718537.
- ↑ Healy D (2004) Let them eat Prozac. New York: New York University Press. pp 124–148.
- ↑ Antidepressants and Violence: Problems at the Interface of Medicine and Law, David Healy, Andrew Herxheimer, and David B. Menkes, PLoS Med 3(9): e372 doi:10.1371/journal.pmed.0030372, 2006
- ↑ Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration" (htm). PLoS Medicine. Retrieved on 2008-02-26.
- ↑ "The creation of the Prozac myth", The Guardian (February 27 2008). Retrieved on 2008-03-01.
- ↑ Day, Michael (2008-02-26). "Prozac does not work in majority of depressed patients", New Scientist. Retrieved on 2008-03-01.
- ↑ Blue, Laura (February 26, 2008). "Antidepressants Hardly Help". Retrieved on 2008-03-01.
External links
- Biography of Dr. David T. Wong, one of the inventors of Prozac
- Biography of Dr. Bryan B. Molloy, one of the inventors of Prozac
- Trouble in Prozac - Fortune Magazine
|
|||||||||||||||||||||||||||||||