Feather

Feathers are one of the epidermal growths that form the distinctive outer covering, or plumage, on birds. They are considered the most complex integumentary structures found in vertebrates.[1] They are among the outstanding characteristics that distinguish the extant Aves from other living groups. Feathers have also been noticed in Theropoda which have been termed Feathered dinosaurs. Although feathers cover most parts of the body of birds, they arise only from certain well-defined tracts on the skin. They aid in flight, thermal insulation, waterproofing and coloration that helps in communication and protection.[2]
Contents |
Structure and characteristics

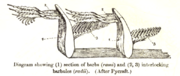
1. Vane
2. Rachis
3. Barb
4. Afterfeather
5. Hollow shaft, calamus
Feathers are among the most complex integumentary appendages found in vertebrates and are formed in tiny follicles in the epidermis, or outer skin layer, that produce keratin proteins. The β-keratins in feathers, beaks and claws — and the claws, scales and shells of reptiles — are composed of protein strands hydrogen-bonded into β-pleated sheets, which are then further twisted and crosslinked by disulfide bridges into structures even tougher than the α-keratins of mammalian hair, horns and hoof.[3][4] The exact signals that induce the growth of feathers on the skin are not known but it has been found that the transcription factor cDermo-1 induces the growth of feathers on skin and scales on the leg.[5]
Classification
- See also: Flight feather
There are two basic types of feather: vaned feathers which cover the exterior of the body, and down feathers which are underneath the vaned feathers. The pennaceous feathers are vaned feathers. Also called contour feathers, pennaceous feathers arise from tracts and cover the whole body. Some feathers, filoplumes, are hairlike and are found along with the down feathers. In some passerines filoplumes arise exposed beyond the contour feathers on the neck.[1] The remiges, or flight feathers of the wing, and rectrices, the flight feathers of the tail are the most important feathers for flight. A typical vaned feather features a main shaft, called the rachis. Fused to the rachis are a series of branches, or barbs; the barbs themselves are also branched and form the barbules. These barbules have minute hooks called barbicels for cross-attachment. Down feathers are fluffy because they lack barbicels, so the barbules float free of each other, allowing the down to trap much air and provide excellent thermal insulation. At the base of the feather, the rachis expands to form the hollow tubular calamus (or quill) which inserts into a follicle in the skin. The basal part of the calamus is without vanes. This part is embedded within the skin follicle and has an opening at the base (proximal umbilicus) and a small opening on the side (distal umbilicus).[6]
Hatchling birds of some species have a special kind of natal down (neossoptiles) and these are pushed out when the normal feathers (teleoptiles) emerge.[1]
Flight feathers are stiffened so as to work against the wind in the downstroke but yield in other directions. It is noted that the pattern of orientation of β-keratin fibers in the feathers of flying birds differs from that in flightless birds. The fibers are better aligned in the middle of the feather and less aligned towards the tips.[7][8]
Functions
Feathers insulate birds from water and cold temperatures. They may also be plucked to line the nest and provide insulation to the eggs and young. The individual feathers in the wings and tail play important roles in controlling flight. Some species have a crest of feathers on their heads. Although feathers are light, a bird's plumage weighs two or three times more than its skeleton, since many bones are hollow and contain air sacs. Color patterns serve as camouflage against predators for birds in their habitats, and by predators looking for a meal. As with fish, the top and bottom colors may be different to provide camouflage during flight. Striking differences in feather patterns and colors are part of the sexual dimorphism of many bird species and are particularly important in selection of mating pairs. In some cases there are differences in the UV reflectivity of feathers across sexes even though no differences in color are noted in the visible range.[9] The wing feathers of male Club-winged Manakins Machaeropterus deliciosus have special structures that are used to produce sounds by stridulation.[10]

Some birds have a supply of powder down feathers which grow continuously, with small particles regularly breaking off from the ends of the barbules. These particles produce a powder that sifts through the feathers on the bird's body and acts as a waterproofing agent and a feather conditioner. Powder down has evolved independently in several taxa and can be found in down as well as pennaceous feathers. They may be scattered in plumage in the pigeons and parrots or in localized patches on the breast, belly or flanks as in herons and frogmouths. Herons use their bill to break the feathers and to spread them while cockatoos may use their head as a powder puff to apply the powder.[11] Waterproofing can be lost by exposure to emulsifying agents due to human pollution. Feathers can become waterlogged and birds may sink. It is also very difficult to clean and rescue birds whose feathers have been fouled by oil spills. The feathers of cormorants soak up water and help in reducing buoyancy and thereby allowing the birds to swim submerged.[12]

Bristles are stiff, tapering feathers with a large rachis but few barbs. Rictal bristles are bristles found around the eyes and bill. They may serve a similar purpose to eyelashes and vibrissae in mammals. It has been suggested that they may aid insectivorous birds in prey capture or that it may have sensory functions, however there is no clear evidence.[13] In one study, Willow Flycatchers (Empidonax traillii) and they were found to catch insects equally well before and after removal of the rictal bristles.[14]
Grebes are peculiar in their habit of ingesting their own feathers and also feeding them to their young. Observations on the diet and feather eating frequency suggest that ingesting feathers particularly down from their flanks aids in forming easily ejectable pellets along with their diet of fish.[15]
Distribution
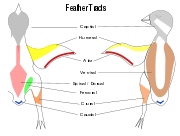
Contour feathers are not uniformly distributed on the skin of the bird except in some groups such as the Penguins, ratites and screamers.[16] In most birds the feathers grow from specific tracts of skin called pterylae while there are regions which are free of feathers called apterylae. Filoplumes and down may arise from the apteriae, regions between the pterylae. The arrangement of these feather tracts, pterylosis or pterylography, varies across bird families and has been used in the past as a means for determining the evolutionary relationships of bird families.[17][18]
Coloration
The colors of feathers are produced by the presence of pigments such as melanins (browns, blacks, greys), carotenoids (reds, yellows, orange), psittacofulvins (unique red pigments found in some parrots)[19] and porphyrins (such as the green turacoverdin of Turacos) or more often by feather structure. Structural coloration is involved in the production of most greens, blues, iridescent colors, ultraviolet reflectance and in the enhancement of pigmentary colors.[20][2][21]
The blues and greens of many parrots are produced by constructive interference of light reflecting from different layers of the structures in feathers in addition to the yellow carotenoid pigments. Melanin is often involved in the absorption of some of the light in these feathers. The specific feather structure involved is sometimes called the Dyck texture.[22][23]

Albinism is caused by the lack of pigment in some or all of a bird's feathers.
In some birds, the feather colors may be created or altered by uropygial gland secretions. The yellow bill colors of many hornbills are produced by preen gland secretions. Other differences that may only be visible in the ultraviolet region have been suggested[11] but studies have failed to find evidence.[24] Uropygial oil secretion may also have an inhibitory effect on feather bacteria.[25]
A bird's feathers undergo wear and tear and are replaced periodically during its life through molting. New feathers are formed through the same follicle from which the old ones were fledged. The presence of melanin in feathers increases their resistance to abrasion.[26] Melanin based feathers were however found to be faster degraded by bacteria than those with carotenoid pigments.[27] This has led to the suggestion that Gloger's rule, the observation that birds from more humid regions tend to be darker may be related to the increased bacterial load and the selection for greater melanin.[28] The evolution of coloration is based on sexual selection and it has been suggested that carotenoid based pigments may have evolved since they are likely to be more honest signals of fitness since they are derived from special diets.[29]
Parasites
The feather surface is the home for some ectoparasites, notably feather lice (Phthiraptera) and feather mites. Feather lice typically live on a single host and can move only from parents to chicks or mating birds and occasionally by phoresy. This life history has resulted in most of the species being specific to the host and coevolving with the host making them of interest in phylogenetic studies.[30] Interesting parasitic cuckoos which grow up in the nests of other species also have host specific feather lice and these seem to be transmitted only after the leave the host nest.[31]
Birds maintain their feather condition by bathing in water, dust bathing and preening. A peculiar behaviour of birds, anting, where ants are introduced into the plumage was suggested to help in reducing parasites but no supporting evidence has been found.[32]
Human usage

Feathers have a number of utilitarian and cultural and religious uses.
Utilitarian functions
Feathers are both soft and excellent at trapping heat; thus, they are sometimes used in high-class bedding, especially pillows, blankets, and mattresses. They are also used as filling for winter clothing, such as quilted coats and sleeping bags; goose and eider down have great loft, the ability to expand from a compressed, stored state to trap large amounts of compartmentalized, insulating air.[33]
Bird feathers have long been used for fletching arrows. Colorful feathers such as those belonging to pheasants have been used to decorate fishing lures.
During the 18th, 19th, and even 20th Centuries a booming international trade in plumes, to satisfy market demand in North America and Europe for extravagant head-dresses as adornment for fashionable women, caused so much destruction (for example, to egret breeding colonies) that a major campaign against it by conservationists led to the Lacey Act and caused the fashion to change and the market to finally collapse. Frank Chapman noted in 1886 that as many as 40 species of birds were used in about three-fourths of the 700 ladies' hats that he observed in New York City.[34][35][36]
Feathers of large birds (most often geese) have been and are used to make quill pens. The word pen itself is derived from the Latin penna for feather.[37] The French nom-de-plume for pen name has a similar origin.
Feathers are also valuable in aiding the identification of species in forensic studies, particularly in bird strikes to aircraft. The ratios of hydrogen isotopes in feathers help in determining the geographic origins of birds.[38] Feathers may also be useful in the non-destructive sampling of pollutants.[39]
The poultry industry produces a large amount of feathers as waste and like other forms of keratin are slow in their decomposition. Feather waste has been used in a number of industrial applications as a medium for culturing microbes,[40] biodegradeable polymers,[41] and production of enzymes.[42] Feather proteins have been tried as an adhesive for wood board.[43]
In religion and culture
Eagle feathers have great cultural and spiritual value to American Indians in the USA and First Nations peoples in Canada as religious objects. In the United States the religious use of eagle and hawk feathers are governed by the eagle feather law, a federal law limiting the possession of eagle feathers to certified and enrolled members of federally recognized Native American tribes.
Various birds and their plumages serve as cultural icons throughout the world, from the hawk in ancient Egypt to the bald eagle and the turkey in the United States. In Greek mythology, Daedelus the inventor and Icarus tried to escape his prison by attaching feathered wings to his shoulders with wax, which was melted by the Sun.
In South America, brews made from the feathers of Condors are used in traditional medications.[44] In India, feathers of the Indian Peacock have been used in traditional medicine for snakebite, infertility and coughs.[45][46]
Evolution
The functional view on the evolution of feathers has traditionally focussed on insulation, flight and display. Discoveries of non-flying Late Cretaceous feathered dinosaurs in China however suggest that flight could not have been the primary function.[47] While feathers have been suggested as being evolved from reptilian scales, there are numerous objections. Scale-based origins of feathers suggest that the planar scale structure was modified by their development into feathers by splitting to form the webbing, however the developmental process involves a tubular structure arising from a follicle and the tube splitting longitudinally to form the webbing.[1] The number of feathers per unit area of skin is higher in smaller birds than in larger birds and this trend indicates their important role in thermal insulation since smaller birds lose more heat due to the relatively larger surface area for their body weight.[2] The coloration of feathers is believed to be primarily evolved in response to sexual selection. In many cases the physiological condition of the birds (especially males) is indicated by the quality of their feathers and this is used(by the females) in mate choice.[48][49]
Feathered dinosaurs
Several dinosaurs had feathers on their limbs that would not have functioned for flight. One theory is that feathers originally developed on dinosaurs as a means of insulation; those small dinosaurs that then grew longer feathers may have found them helpful in gliding leading to the evolution of proto-birds like Archaeopteryx and Microraptor zhaoianus. Sinosauropteryx had short fibres and symmetrical feather like structures are seen in Protarchaeopteryx and Caudipteryx.[47] Other dinosaurs that had feathers or protofeathers include Pedopenna daohugouensis, [50] and Dilong paradoxus, a tyrannosauroid which is 60 to 70 million years older than Tyrannosaurus rex.[51]
See also
- Pinioning
References
- ↑ 1.0 1.1 1.2 1.3 Prum, Richard O. & AH Brush (2002). "The evolutionary origin and diversification of feathers". The Quarterly Review of Biology 77 (3): 261–295. doi:.
- ↑ 2.0 2.1 2.2 Pettingill, OS Jr. (1970). Ornithology in Laboratory and Field. Fourth edition. Burgess Publishing Company. pp. 29–58. ISBN 808716093.
- ↑ R. Schor and S. Krimm (1961). "Studies on the Structure of Feather Keratin II. A β-Helix Model for the Structure of Feather Keratin". Biophys J. 1 (6): 489–515.
- ↑ Linus Pauling and Robert B. Corey (1951). "The Structure of Feather Rachis keratin". Proceedings of the National Academy of Sciences of the United States of America 37 (5): 256–261. doi:. PMID 14834148.
- ↑ Hornik, C., Krishan, K., Yusuf, F., Scaal, M., & Brand-Saberi, B. (2005). "cDermo-1 misexpression induces dense dermis, feathers, and scales". Developmental Biology 277 (1): 42–50. doi:.
- ↑ McLelland, J. (1991). A color atlas of avian anatomy. W.B. Saunders Co..
- ↑ Cameron, G., Wess, T., & Bonser, R. (2003). "Young’s modulus varies with differential orientation of keratin in feathers". Journal of Structural Biology 143 (2): 118. doi:.
- ↑ Bonser, R., Saker, L., & Jeronimidis, G. (2004). "Toughness anisotropy in feather keratin". Journal of Materials Science 39 (8): 2895–2896. doi:.
- ↑ Muir D. Eaton and Scott M. Lanyon (2003). "The Ubiquity of Avian Ultraviolet Plumage Reflectance". Proceedings: Biological Sciences 270 (1525): 1721–1726. doi:.
- ↑ Bostwick, Kimberly S. and Richard O. Prum (2005). "Courting Bird Sings with Stridulating Wing Feathers". Science 309 (5735): 736. doi:. PMID 16051789.
- ↑ 11.0 11.1 Delhey K, A. Peters, and B. Kempenaers (2007). "Cosmetic coloration in birds: occurrence, function and evolution" (PDF). Am. Nat. 169: S145–158. doi:. http://www.orn.mpg.de/documents/peters/Delhey_AmNat2007_copy.pdf.
- ↑ Ribak, G., Weihs, D. and Arad, Z. (2005). "Water retention in the plumage of diving great cormorants Phalacrocorax carbo sinensis". J. Avian Biol. 36: 89–95. doi:.
- ↑ Lederer R. J. (1972) The role of avian rictal bristles. Wilson. Bull. 84, 193-97 pdf
- ↑ Conover, M. R., and D. E. Miller (1980) Rictal bristle function in willow flycatcher. Condor 82:469-471.
- ↑ Piersma, T & M R van Eerden (1989). "Feather eating in Great Crested Grebes Podiceps cristatus: a unique solution to the problems of debris and gastric parasites in fish-eating birds". Ibis 131 (4): 477–486. doi:.
- ↑ Demay, Ida S. (1940). "A Study of the Pterylosis and Pneumaticity of the Screamer". The Condor 42 (2): 112–118. doi:.
- ↑ K. Susanna S. Hall (2005). "Do nine-primaried passerines have nine or ten primary feathers? The evolution of a concept". Journal of Ornithology 146 (2): 121–126. doi:.
- ↑ Pycraft, W. P. (1895). "On the pterylography of the hoatzin (Opisthocomus cristatus)". Ibis 37: 345–373. doi:.
- ↑ McGraw KH & MC Nogare (2005). "Distribution of unique red feather pigments in parrots". Biology Letters 1 (1): 38–43. doi:.
- ↑ Hausmann, F., Arnold, K.E., Marshall, N.J. & Owens, I.P.F. (2003). "Ultraviolet signals in birds are special". Proc. R. Soc. B 270: 61–67. doi:.
- ↑ Matthew D Shawkey and Geoffrey E Hill (2005). "Carotenoids need structural colours to shine" (PDF). Biol Lett. 1 (2): 121–124. doi:. http://nature.berkeley.edu/%7Emshawkey/9.pdf.
- ↑ Dyck J. (1971). "Structure and spectral reflectance of green and blue feathers of the Lovebird (Agapornis roseicollis)". Biol. Skr. 18: 1–67.
- ↑ Shawkey MD & G E Hill (2005). "Feathers at a fine scale" (PDF). The Auk 121 (3): 652–655. doi:. http://nature.berkeley.edu/%7Emshawkey/6.pdf.
- ↑ Delhey, K., A. Peters, PHW Biedermann & B Kempenaers (2008). "Optical properties of the uropygial gland secretion: no evidence for UV cosmetics in birds". Naturwissenschaften 95: 939. doi:.
- ↑ Shawkey, M.D., S.R. Pillai, and G.E. Hill (2003). "Chemical warfare? Effects of uropygial oil on feather-degrading bacteria" (PDF). Journal of Avian Biology 34: 345–349. doi:. http://nature.berkeley.edu/~mshawkey/2.pdf.
- ↑ Bonser, R. H. C. (1995). "Melanin and the abrasion resistance of feathers". Condor 97: 590–591. doi:.
- ↑ Grande JM, Negro JJ & MJ Torres (2004). "The evolution of bird plumage colouration: A role for feather-degrading bacteria?" (PDF). Ardeola 51 (2): 375–383. http://www.ardeola.org/files/Ardeola_51(2)_375-383.pdf.
- ↑ Burtt, Edward H. Jr. & Ichida, Jann M. (2004). "Gloger's Rule, feather-degrading bacteria, and color variation among Song Sparrows" (PDF). Condor 106 (3): 681–686. doi:. http://www.public.asu.edu/~kjmcgraw/pubs/Condor04b.pdf.
- ↑ Badyaev AV & Hill GE (2000). "Evolution of sexual dichromatism: contribution of carotenoid versus melanin-based colouration". Biological Journal of the Linnean Society 69: 153–172. doi:.
- ↑ Toon, A., & Hughes, J. (2008). "Are lice good proxies for host history? A comparative analysis of the Australian magpie, Gymnorhina tibicen, and two species of feather louse". Heredity 101 (2): 127–135. doi:.
- ↑ Brooke, M. de L. and Hiroshi Nakamura (1998). "The acquisition of host-specific feather lice by common cuckoos (Cuculus canorus)". Journal of Zoology 244: 167–173. doi:.
- ↑ Revis, Hannah C., and Deborah A. Waller (2004). "Bactericidal and fungicidal activity of ant chemicals on feather parasites: an evaluation of anting behavior as a method of self-medication in songbirds". Auk 121 (4): 1262–1268. doi:.
- ↑ Bonser, R.H.C. & Dawson, C. (1999). "The structural mechanical properties of down feathers and biomimicking natural insulation materials". Journal of Materials Science Letter 18 (21): 1769–1770. doi:.
- ↑ Doughty, Robin W. 197. Feather Fashions and Bird Preservation, A Study in Nature Protection. University of California Press.
- ↑ Ehrlich, Paul R.; Dobkin. David S.; Wheye. Darryl (1988) Plume Trade] Stanford University
- ↑ Feather trade Smithsonian Institution
- ↑ The American Heritage Dictionary of the English Language, Fourth Edition. 2000. Houghton Mifflin Company. [1]
- ↑ Bowen, Gabriel J.;Leonard I. Wassenaar;Keith A. Hobson (2005). "Global application of stable hydrogen and oxygen isotopes to wildlife forensics". Oecologia 143 (3): 337–348. doi:.
- ↑ Jaspers, V., Voorspoels, S., Covaci, A., Lepoint, G., & Eens, M. (2007). "Evaluation of the usefulness of bird feathers as a non-destructive biomonitoring tool for organic pollutants: A comparative and meta-analytical approach". Environment International 33 (3): 328–337. doi:.
- ↑ Subbiah Poopathi, S. Abidha (2007). "Use of feather-based culture media for the production of mosquitocidal bacteria". Biological Control 43 (1): 49–55. doi:.
- ↑ Schmidt, W.F., Barone, J.R. (2004). New uses for chicken feathers keratin fiber. Poultry Waste Management Symposium Proceedings. pp. 99–101.
- ↑ Casarin, Franciani; Florencia Cladera-Olivera & Adriano Brandelli (2008). "Use of Poultry Byproduct for Production of Keratinolytic Enzymes". Food and Bioprocess Technology 1 (3): 301–305. doi:.
- ↑ Jiang, Z., Qin, D., Hse, C., Kuo, M., Luo, Z., Wang, G., et al. (2008). "Preliminary Study on Chicken Feather Protein-Based Wood Adhesives". Journal of Wood Chemistry & Technology 28 (3): 240–246. doi:.
- ↑ Steve Froemming (2006). "Traditional use of the Andean flicker (Colaptes rupicola) as a galactagogue in the Peruvian Andes". Journal of Ethnobiology and Ethnomedicine 2: 23. doi:.
- ↑ Murari, S.K., Frey, F.J., Frey, B.M., Gowda, T.V., Vishwanath, B.S. (2005). "Use of Pavo cristatus feather extract for the better management of snakebites: Neutralization of inflammatory reactions". Journal of Ethnopharmacology 99 (2): 229–237. doi:.
- ↑ Mahawar, MM & DP Jaroli (2007). "Traditional knowledge on zootherapeutic uses by the Saharia tribe of Rajasthan, India". J Ethnobiol Ethnomedicine 3: 25. doi:.
- ↑ 47.0 47.1 Sumida, SS & CA Brochu (2000). "Phylogenetic context for the origin of feathers". American Zoologist 40 (4): 486–503. doi:. http://icb.oxfordjournals.org/cgi/content/abstract/40/4/486.
- ↑ Saino, Nicola, and Riccardo Stradi (1999). "Carotenoid Plasma Concentration, Immune Profile, and Plumage Ornamentation of Male Barn Swallows". American Naturalist 154 (4): 441. doi:.
- ↑ Endler, John A., David A. Westcott, Joah R. Madden, Tim Robson, and Patrick Phillips (2005). "Animal visual systems and the evolution of color patterns: Sensory processing illumiates signal evolution". Evolution 59 (8): 1795–1818.
- ↑ Xu, Xing & Fucheng Zhang (2005). "A new maniraptoran dinosaur from China with long feathers on the metatarsus". Naturwissenschaften 92 (4): 173–177. doi:.
- ↑ Xu, Xing (2006). "Feathered dinosaurs from China and the evolution of major avian characters". Integrative Zoology 1 (1): 4–11. doi:.
External links
- McGraw, K. J. 2005. Polly want a pigment? Cracking the chemical code to red coloration in parrots. Australian Birdkeeper Magazine 18:608-611.
- DeMeo, Antonia M. Access to Eagles and Eagle Parts: Environmental Protection v. Native American Free Exercise of Religion (1995) [2]
- Electronic Code of Federal Regulations (e-CFR), Title 50: Wildlife and Fisheries PART 22—EAGLE PERMITS [3]
- U.S. v. Thirty Eight Golden Eagles (1986) [4]
- Mechanical structure of feathers
- Scans 250 species.Excellent images.
|
||||||||||||||||||||||||||
|
|||||
