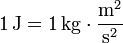Joule
For the physicist named James Prescott Joule, see James Prescott Joule.
The Joule is the derived unit of energy in the International System of Units. It is defined as:
One joule is the amount of energy required to perform the following actions:
- The work done by a force of one newton travelling through a distance of one meter;
- The work required to move an electric charge of one coulomb through an electrical potential difference of one volt; or one coulomb volt, with the symbol C·V;
- The work done to produce power of one watt continuously for one second; or one watt second (compare kilowatt hour), with the symbol W·s. Thus a kilowatt hour is 3,600,000 joules or 3.6 megajoules;
- The kinetic energy of a 2 kg mass moving at a velocity of 1 m/s. The energy is linear in the mass but quadratic in the velocity, being given by E = ½mv²;
Contents |
Conversions
1 joule is exactly 107 ergs.
1 joule is approximately equal to:
- 6.2415 ×1018 eV (electronvolts)
- 0.2390 cal (calorie) (small calories, lower case c)
- 2.3901 ×10−4 kilocalorie, Calories (food energy, upper case C)
- 9.4782 ×10−4 BTU (British thermal unit)
- 0.7376 ft·lbf (foot-pound force)
- 23.7 ft·pdl (foot poundals)
- 2.7778 ×10−7 kilowatt hour
- 2.7778 ×10−4 watt hour
- 9.8692 ×10−3 litre-atmosphere
Units defined in terms of the joule include:
- 1 thermochemical calorie = 4.184 J
- 1 International Table calorie = 4.1868 J
- 1 watt hour = 3600 J
- 1 kilowatt hour = 3.6 ×106 J (or 3.6 MJ)
- 1 ton TNT = 4.184 GJ
Useful to remember:
- 1 joule = 1 newton meter = 1 watt second
Practical examples
One joule in everyday life is approximately:
- the energy required to lift a small apple one meter straight up.
- the energy released when that same apple falls one meter to the ground.
- the energy released as heat by a quiet person, every hundredth of a second.
- the energy required to heat one gram of dry, cool air by 1 degree Celsius.
- one hundredth of the energy a person can receive by drinking a drop of beer.
- the kinetic energy of an adult human moving a distance of about a handspan every second.
SI multiples
| Submultiples | Multiples | |||||
|---|---|---|---|---|---|---|
| Value | Symbol | Name | Value | Symbol | Name | |
| 10–1 J | dJ | decijoule | 101 J | daJ | decajoule | |
| 10–2 J | cJ | centijoule | 102 J | hJ | hectojoule | |
| 10–3 J | mJ | millijoule | 103 J | kJ | kilojoule | |
| 10–6 J | µJ | microjoule | 106 J | MJ | megajoule | |
| 10–9 J | nJ | nanojoule | 109 J | GJ | gigajoule | |
| 10–12 J | pJ | picojoule | 1012 J | TJ | terajoule | |
| 10–15 J | fJ | femtojoule | 1015 J | PJ | petajoule | |
| 10–18 J | aJ | attojoule | 1018 J | EJ | exajoule | |
| 10–21 J | zJ | zeptojoule | 1021 J | ZJ | zettajoule | |
| 10–24 J | yJ | yoctojoule | 1024 J | YJ | yottajoule | |
| Common multiples are in bold face | ||||||
| This SI unit is named after James Prescott Joule. As with every SI unit whose name is derived from the proper name of a person, the first letter of its symbol is uppercase (J). When an SI unit is spelled out in English, it should always begin with a lowercase letter (joule), except where any word would be capitalized, such as at the beginning of a sentence or in capitalized material such as a title. Note that "degree Celsius" conforms to this rule because the "d" is lowercase.
— Based on The International System of Units, section 5.2.
|
See also
- Conversion of units
- SI prefixes
- Orders of magnitude
- Orders of magnitude (energy)
- Electronvolt
- Kilowatt hour
- Fluence
- Foe
References
- The adoption of joules as units of energy, FAO/WHO Ad Hoc Committee of Experts on Energy and Protein, 1971. A report on the changeover from calories to joules in nutrition.