Evolutionary history of life
| Part of the Biology series on |
| Evolution |
 |
| Introduction |
| Mechanisms and processes |
|
Adaptation |
| Research and history |
|
Evidence |
| Evolutionary biology fields |
|
Cladistics |
| Biology Portal · |
The evolutionary history of life on Earth stretches back over , possibly as far as , and there is evidence that evolution continues, even in humans. All present-day organisms use the same large set of complex chemical reactions, which indicates that all modern organisms share a common ancestor. However even the simplest modern organisms are too complex to have emerged directly from non-living materials. Some scientists have proposed that life on Earth was "seeded" from elsewhere, but most research concentrates on various explanations of how life could have arisen independently on Earth.
For about 2,000 million years multi-layered microbial mats were the dominant life on Earth. The evolution of oxygenic photosynthesis led to the oxygenation of the atmosphere, beginning about . While eucaryotic cells may have been present earlier, their evolution accelerated when they acquired the ability to use oxygen as a powerful "fuel" for their metabolisms. The earliest evidence of more complex eucaryotes with organelles, "organs" within a cell, dates from . Multicellular life is composed only of eucaryotic cells, and the earliest evidence for it is from , although specialization of cells for different functions first appears between ( a possible fungus) and (a probable red alga). Sexual reproduction may be a prerequisite for specialization of cells.
The earliest known fossil animals are cnidarians from about , although the earliest animals must have appeared before then. The earliest modern-looking bilaterian animals appear in the Early Cambrian, along with several "weird wonders". There is a long-running debate about whether this Cambrian explosion was truly a very rapid period of evolutionary experimentation. Vertebrates remained an obscure group until the first fish with jaws appeared in the Late Ordovician.
The earliest signs of land plants and land invertebrates date back to about and respectively. The lineage that produced land vertebrates evolved very rapidly between and , and acquired limbs while they were still wholly aquatic. Land plants were so successful that they caused an ecological crisis in the Late Devonian.
During the Permian period synapsids, including the ancestors of mammals, may have dominated land environments, but the Permian–Triassic extinction event came very close to wiping out complex life. During the slow recovery from this catastrophe, archosaurs became the most abundant terrestrial vertebrates. One archosaur group, the dinosaurs, were the dominant land vertebrates in the Jurassic and Cretaceous periods, and birds evolved from one group of dinosaurs. During this time mammals' ancestors could survive only as small, mainly nocturnal insectivores, but this may have accelerated the development of mammalian traits. After the Cretaceous–Tertiary extinction event killed off the non-avian dinosaurs – birds are the only surviving dinosaurs – mammals increased rapidly in size and diversity, and some took to the air and the sea.
Fossil evidence indicates that flowering plants appeared and rapidly diversified in the Early Cretaceous, between and , probably helped by coevolution with pollinating insects. Flowering plants and marine phytoplankton are still the dominant producers of organic matter. Social insects appeared around the same time as flowering plants. Although they occupy only small parts of the insect "family tree", they now form over half the total mass of insects.
Humans evolved from a lineage of upright-walking apes whose earliest fossils date from over . Although early members of this lineage had chimp-sized brains, there are signs of a steady increase in brain size after about .
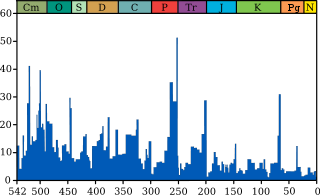
The mass extinctions that have occurred at least since may have accelerated the evolution of life by making room for new groups of organisms to diversify. The fossil record shows a swift rise in biodiversity from , a slight decline from , and a swift rise from to the present.
Earliest history of Earth
Millions of years
The oldest meteorite fragments found on Earth are about , and this has convinced scientists that the whole Solar system, including Earth, formed around then.[1] About 40 million years later a planetoid struck the Earth, throwing into orbit the material that formed the Moon.[2]
Until recently the oldest rocks found on Earth were about ,[1] and this led scientists to believe for decades that Earth's surface was molten until then. Hence they named this part of Earth's history the Hadean eon, whose name means "hellish". [3] However analysis of zircons formed indicates that Earth's crust solidified about 100 million years after the planet's formation and that Earth quickly acquired oceans and an atmosphere, which may have been capable of supporting life.[4]
Evidence from the Moon indicates that from it suffered a Late Heavy Bombardment by debris that was left over from the formation of the Solar system, and Earth, having stronger gravity, should have experienced an even heavier bombardment.[5][3] While there is no direct evidence of conditions on Earth , there is no reason to think that the Earth was not also affected by this late heavy bombardment.[6] This event may well have stripped away any previous atmosphere and oceans; in this case gases and water from comet impacts may have contributed to their replacement, although volcanic outgassing on Earth would have contributed at least half.[7]
Earliest evidence for life on Earth
The earliest organisms were minute and relatively featureless, so their fossils look like small rods, which are very difficult to tell apart from structures which form through physical processes. The oldest undisputed evidence of life on Earth, interpreted as fossilized bacteria, dates to .[8] Other finds in rocks dated to about have been interpreted as bacteria,[9] and geochemical evidence seemed to show the presence of life .[10] However these analyses were closely scrutinised, and non-biological processes were found which could produce all of the "signatures of life" that had been reported.[11][12] While this does not prove that the structures found had a non-biological origin, they cannot be taken as clear evidence for the presence of life. Currently, the oldest unchallenged evidence for life is geochemical signatures from rocks deposited ,[8][13] although there has been little time for these recent reports (2006) to be examined by critics.
Origins of life on Earth
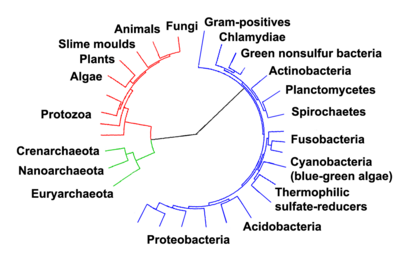
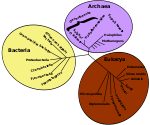
- For more details on this topic, see Evidence of common descent, Common descent, and Homology (biology).
Biochemists reason that all living organisms on Earth must share a single Last Universal Common Ancestor, because it would be unbelievable that two or more separate lineages could have independently developed the many complex biochemical mechanisms shared by all living organisms.[15][16] However the earliest organisms for which fossil evidence is available are bacteria, which are far too complex to have arisen directly from non-living materials.[17] The lack of fossil or geochemical evidence for earlier types of organism has left plenty of scope for hypotheses, which fall into two main groups: that life arose spontaneously on Earth, and that it was "seeded" from elsewhere in the universe.
Life "seeded" from elsewhere
The idea that life Earth was "seeded" from elsewhere in the universe dates back at least to the fifth century BC.[18] In the twentieth century it was proposed by the physical chemist Svante Arrhenius,[19] by the astronomers Fred Hoyle and Chandra Wickramasinghe,[20] and by molecular biologist Francis Crick and chemist Leslie Orgel.[21] There are three main versions of the "seeded from elsewhere" hypothesis: from elsewhere in our Solar system via fragments knocked into space by a large meteor impact, in which case the only credible source is Mars;[22] by alien visitors, possibly as a result of accidental contamination by micro-organisms that they brought with them;[21] and from outside the Solar system but by natural means.[22][19] Experiments suggest that some micro-organisms can survive the shock of being catapulted into space and some can survive exposure to radiation for several days, but there is no proof that they can survive in space for much longer periods.[22] Scientists are divided over the likelihood of life arising independently on Mars,[23] or on other planets in our galaxy.[22]
Independent emergence on Earth
Research on how life might have emerged unaided from non-living chemicals focuses on three possible starting points: self-replication, an organism's ability to produce offspring that are very similar to itself; metabolism, its ability to feed and repair itself; and external cell membranes, which allow food to enter and waste products to leave, but exclude unwanted substances.[24] Research on abiogenesis still has a long way to go, since theoretical and empirical approaches are only beginning to make contact with each other.[25][26]
Replication first: RNA world
![The replicator in virtually all known life is deoxyribonucleic acid. DNA's structure and replication systems are far more complex than those of the original replicator.[17]](/2009-wikipedia_en_wp1-0.7_2009-05/I/200px_DNA_replication_split_svg.png)
Even the simplest members of the three modern domains of life use DNA to record their "recipes" and a complex array of RNA and protein molecules to "read" these instructions and use them for growth, maintenance and self-replication. This system is far too complex to have emerged directly from non-living materials.[17] The discovery that some RNA molecules can catalyze both their own replication and the construction of proteins led to the hypothesis of earlier life-forms based entirely on RNA.[27] These ribozymes could have formed an RNA world in which there were individuals but no species, as mutations and horizontal gene transfers would have meant that the offspring in each generation were quite likely to have different genomes from those that their parents started with.[28] RNA would later have been replaced by DNA, which is more stable and therefore can build longer genomes, expanding the range of capabilities a single organism can have.[28][29][30] Ribozymes remain as the main components of ribosomes, modern cells' "protein factories".[31]
Although short self-replicating RNA molecules have been artificially produced in laboratories,[32] doubts have been raised about where natural non-biological synthesis of RNA is possible.[33] The earliest "ribozymes" may have been formed of simpler nucleic acids such as PNA, TNA or GNA, which would have been replaced later by RNA.[34][35]
In 2003 it was proposed that porous metal sulfide precipitates would assist RNA synthesis at about 100 °C (212 °F) and ocean-bottom pressures near hydrothermal vents. In this hypothesis lipid membranes would be the last major cell components to appear and until then the proto-cells would be confined to the pores.[36]
Metabolism first: Iron-sulfur world
A series of experiments starting in 1997 showed that early stages in the formation of proteins from inorganic materials including carbon monoxide and hydrogen sulfide could be achieved by using iron sulfide and nickel sulfide as catalysts. Most of the steps required temperatures of about 100 °C (212 °F) and moderate pressures, although one stage required 250 °C (482 °F) and a pressure equivalent to that found under 7 kilometres (4.3 mi) of rock. Hence it was suggested that self-sustaining synthesis of proteins could have occurred near hydrothermal vents.[37]
Membranes first: Lipid world
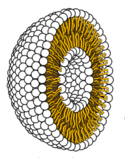
= water-attracting heads of lipid molecules
= water-repellent tails
It has been suggested that double-walled "bubbles" of lipids like those that form the external membranes of cells may have been an essential first step.[38] Experiments that simulated the conditions of the early Earth have reported the formation of lipids, and these can spontaneously form liposomes, double-walled "bubbles", and then reproduce themselves. Although they are not intrinsically information-carriers as nucleic acids are, they would be subject to natural selection for longevity and reproduction. Nucleic acids such RNA might then have formed more easily within the liposomes than they would have outside.[39]
The clay theory
RNA is complex and there are doubts about whether it can be produced non-biologically in the wild.[33] Some clays, notably montmorillonite, have properties that make them plausible accelerators for the emergence of an RNA world: they grow by self-replication of their crystalline pattern; they are subject to an analog of natural selection, as the clay "species" that grows fastest in a particular environment rapidly becomes dominant; and they can catalyze the formation of RNA molecules.[40] Although this idea has not become the scientific consensus, it still has active supporters.[41]
Research in 2003 reported that montmorillonite could also accelerate the conversion of fatty acids into "bubbles", and that the "bubbles" could encapsulate RNA attached to the clay. These "bubbles" can then grow by absorbing additional lipids and then divide. The formation of the earliest cells may have been aided by similar processes.[42]
A similar hypothesis presents self-replicating iron-rich clays as the progenitors of nucleotides, lipids and amino acids.[43]
Environmental and evolutionary impact of microbial mats

These multi-layered colonies of bacteria and other organisms are generally only a few millimeters thick, but still contain a wide range of chemical environments.[44] In modern underwater mats the top layer often consists of photosynthesizing cyanobacteria which create an oxygen-rich environment, while the bottom layer is oxygen-free and often dominated by hydrogen sulfide emitted by the organisms living there. To some extent each mat forms its own food chain, as the by-products of each group of micro-organisms generally serve as "food" for adjacent groups.[45]
Stromatolites are stubby pillars built as microbes in mats slowly migrate upwards to avoid being smothered by sediment deposited on them by water.[44] Although earlier reports of fossilized stromatolites from about were criticized on the grounds that the structures in the rocks could have been produced by non-biological processes,[11] in 2006 another find of stromatolites was reported from the same part of Australia, in rocks also dated to .[46]
It is estimated that the appearance of oxygenic photosynthesis by bacteria in mats increased biological productivity by a factor of between 100 and 1,000. The reducing agent used by oxygenic photosynthesis is water, which is much more plentiful than the geologically-produced reducing agents required by the earlier non-oxygenic photosynthesis.[47] From this point onwards life itself produced significantly more of the resources it needed than did geochemical processes.[48] Oxygen is toxic to organisms that are not adapted to it, but greatly increases the metabolic efficiency of oxygen-adapted organisms.[49][50]
Oxygen became a significant component of Earth's atmosphere about .[51] Although eucaryotes may have been present much earlier,[52][53] the oxygenation of the atmosphere was a prerequisite for the evolution of the most complex eucaryotic cells, from which all multicellular organisms are built.[54] The boundary between oxygen-rich and oxygen-free layers in microbial mats would have moved upwards when photosynthesis shut down overnight, and then downwards as it resumed on the next day. This would have created selection pressure for organisms in this intermediate zone to acquire the ability to tolerate and then to use oxygen, possibly via endosymbiosis, where one organism lives inside another and both of them benefit from their association.[55]
Cyanobacteria have the most complete biochemical "toolkits" of all the mat-forming organisms. Hence they are the most self-sufficient of the mat organisms and were well-adapted to strike out on their own both as floating mats and as the first of the phytoplankton, providing the basis of most marine food chains.[55]
Diversification of eucaryotes
| Eucaryotes |
|
||||||||||||||||||||||||||||||||||||
Eucaryotes may have been present long before the oxygenation of the atmosphere,[52] but most modern eucaryotes require oxygen, which their mitochondria use to fuel the production of ATP, the internal energy supply of all known cells.[54] In the 1970s it was proposed and, after much debate, widely accepted that eucaryotes emerged as a result of a sequence of endosymbioses between "procaryotes". For example: a predatory micro-organism invaded a large procaryote, probably an archaean, but the attack was neutralized, and the attacker took up residence and evolved into the first of the mitochondria; one of these chimeras later tried to swallow a photosynthesizing cyanobacterium, but the victim survived inside the attacker and the new combination became the ancestor of plants; and so on. After each endosymbiosis began, the partners would have eliminated unproductive duplication of genetic functions by re-arranging their genomes, a process which sometimes involved transfer of genes between them.[58][59][60] Another hypothesis proposes that mitochondria were originally sulfur- or hydrogen-metabolising endosymbionts, and became oxygen-consumers later.[61] On the other hand mitochondria might have been part of eucaryotes' original equipment.[62]
There is a debate about when eukaryotes first appeared: the presence of steranes in Australian shales may indicate that eukaryotes were present ;[53] however an analysis in 2008 concluded that these chemicals infiltrated the rocks less than and prove nothing about the origins of eukaryotes.[63] Fossils of the alga Grypania have been reported in rocks (originally dated to but later revised[64]), and indicates that eucaryotes with organelles had already evolved.[65] A diverse collection of fossil algae were found in rocks dated between and .[66] The earliest known fossils of fungi date from .[67]
Multicellular organisms and sexual reproduction
Multicellularity

The simplest definitions of "multicellular", for example "having multiple cells", could include colonial cyanobacteria like Nostoc. Even a professional biologist's definition such as "having the same genome but different types of cell" would still include some genera of the green alga Volvox, which have cells that specialize in reproduction.[69] Multicellularity evolved independently in organisms as diverse as sponges and other animals, fungi, plants, brown algae, cyanobacteria, slime moulds and myxobacteria.[70][64] For the sake of brevity this article focusses on the organisms that show the greatest specialization of cells and variety of cell types, although this approach to the evolution of complexity could be regarded as "rather anthropocentric".[71]
The initial advantages of multicellularity may have included: increased resistance to predators, many of which attacked by engulfing; the ability to resist currents by attaching to a firm surface; the ability to reach upwards to filter-feed or to obtain sunlight for photosynthesis;[72] and even the opportunity for a group of cells to behave "intelligently" by sharing information.[68] These features would also have provided opportunities for other organisms to diversify, by creating more varied environments than flat microbial mats could.[72]
Multicellularity with differentiated cells is beneficial to the organism as a whole but disadvantageous from the point of view of individual cells, most of which lose the opportunity to reproduce themselves. In an asexual multicellular organism, rogue cells which retain the ability to reproduce may take over and reduce the organism to a mass of undifferentiated cells. Sexual reproduction eliminates such rogue cells from the next generation and therefore appears to be a prerequisite for complex multicellularity.[72]
The available evidence indicates that eucaryotes evolved much earlier but remained inconspicuous until a rapid diversification around . The only respect in which eucaryotes clearly surpass bacteria and archaea is their capacity for variety of forms, and sexual reproduction enabled eucaryotes to exploit that advantage by producing organisms with multiple cells that differed in form and function.[72]
How sex evolved
The following hypotheses attempt to explain how and why sex evolved:
- It may have enabled organisms to repair genetic damage.[73] The most primitive form of sex may have been one organism repairing damaged DNA by replicating an undamaged strand from a similar organism.[74]
- Sexual reproduction may have originated from selfish parasitic genetic elements propagating themselves by transfer to new hosts.[75]
- It may have evolved from cannibalism, where some of the victim's DNA was incorporated into the cannibal organism.[74]
- Sexual reproduction may evolved from ancient haloarchaea through a combination of jumping genes, and swapping plasmids.[76]
- Or it may have evolved as a form of vaccination in which infected hosts exchanged weakened symbiotic copies of parasitic DNA as protection against more virulent versions. The meiosis stage of sexual reproduction may then have evolved as a way of removing the symbiotes.[77]

Bacteria also exchange DNA by bacterial conjugation, the benefits of which include resistance to antibiotics and other toxins, and the ability to utilize new metabolites.[78] However conjugation is not a means of reproduction and is not limited to members of the same species, and there are cases where bacteria transfer DNA to plants and animals.[79] Nevertheless it may be an example of the "selfish genetic element" hypothesis, as it transfers DNA by means of such a "selfish gene", the F-plasmid.[74]
Fossil evidence for multicellularity and sexual reproduction
The earliest known fossil organism that is clearly multicellular, Qingshania,[note 1], dated to , appears to consist of virtually identical cells. A red alga called Bangiomorpha, dated at , is the earliest known organism which has differentiated, specialized cells, and is also the oldest known sexually-reproducing organism.[72] The fossils interpreted as fungi appear to have been multicellular with differentiated cells.[67] The "string of beads" organism Horodyskia, found in rocks dated from to , may have been an early metazoan;[64] however it has also been interpreted as a colonial foraminiferan.[80]
Emergence of animals
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
Animals are multicellular eucaryotes,[note 2] and are distinguished from plants, algae, and fungi by lacking cell walls.[82] All animals are motile,[83] if only at certain life stages. All animals except sponges have bodies differentiated into separate tissues, including muscles, which move parts of the animal by contracting, and nerve tissue, which transmits and processes signals.[84]
The earliest widely-accepted animal fossils are rather modern-looking cnidarians (the group that includes jellyfish, sea anemones and hydras), possibly from around , although fossils from the Doushantuo Formation can only be dated approximately. Their presence implies that the cnidarian and bilaterian lineages had already diverged.[85]
The Ediacara biota, which flourished for the last 40 million years before the start of the Cambrian,[86] were the first animals more than a very few centimeters long. Many were flat and had a "quilted" appearance, and seemed so strange that there was a proposal to classify them as a separate kingdom, Vendozoa.[87] Others, however, been interpreted as early molluscs (Kimberella[88][89]), echinoderms (Arkarua[90]), and arthropods (Spriggina,[91] Parvancorina[92]). There is still debate about the classification of these specimens, mainly because the diagnostic features which allow taxonomists to classify more recent organisms, such as similarities to living organisms, are generally absent in the Ediacarans.[93] However there seems little doubt that Kimberella was at least a triploblastic bilaterian animal, in other words significantly more complex than cnidarians.[93]
The small shelly fauna are a very mixed collection of fossils found between the Late Ediacaran and Mid Cambrian periods. The earliest, Cloudina, shows signs of successful defense against predation and may indicate the start of an evolutionary arms race. Some tiny Early Cambrian shells almost certainly belonged to molluscs, while the owners of some "armor plates", Halkieria and Microdictyon, were eventually identified when more complete specimens were found in Cambrian lagerstätten that preserved soft-bodied animals.[94]

In the 1970s there was already a debate about whether the emergence of the modern phyla was "explosive" or gradual but hidden by the shortage of Pre-Cambrian animal fossils.[94] A re-analysis of fossils from the Burgess Shale lagerstätte increased interest in the issue when it revealed animals, such as Opabinia, which did not fit into any known phylum. At the time these were interpreted at evidence that the modern phyla had evolved very rapidly in the "Cambrian explosion" and that the Burgess Shale's "weird wonders" showed that the Early Cambrian was a uniquely experimental period of animal evolution.[95] Later discoveries of similar animals and the development of new theoretical approaches led to the conclusion that many of the "weird wonders" were evolutionary "aunts" or "cousins" of modern groups[96] – for example that Opabinia was a member of the lobopods, a group which includes the ancestors of the arthropods, and that it may have have been closely related to the modern tardigrades.[97] Nevertheless there is still much debate about whether the Cambrian explosion was really explosive and, if so, how and why it happened and why it appears unique in the history of animals.[98]

Most of the animals at the heart of the Cambrian explosion debate are protostomes, one of the two main groups of complex animals. One deuterostome group, the echinoderms, many of which have hard calcite "shells", are fairly common from the Early Cambrian small shelly fauna onwards.[94] Other deuterostome groups are soft-bodied, and most of the significant Cambrian deuterostome fossils come from the Chengjiang fauna, a lagerstätte in China.[99] The Chengjiang fossils Haikouichthys and Myllokunmingia appear to be true vertebrates,[100] and Haikouichthys had distinct vertebrae, which may have been slightly mineralized.[101] Vertebrates with jaws, such as the Acanthodians, first appeared in the Late Ordovician.[102]
Colonization of land
Adaptation to life on land is a major challenge: all land organisms need to avoid drying-out and all those above microscopic size have to resist gravity; respiration and gas exchange systems have to change; reproductive systems cannot depend on water to carry eggs and sperm towards each each other.[103][104]
Plants and the Late Devonian wood crisis


In aquatic algae, almost all cells are capable of photosynthesies and are nearly independent. Life on land required plants to become internally more complex and specialized: photosynthesis was most efficient at the top; roots were required in order to extract water from the ground; the parts in between became supports and transport systems for water and nutrients.[103][105]
Spores of land plants, possibly rather like liverworts, have been found in Mid Ordovician rocks dated to about . In Mid Silurian rocks there are fossils of actual plants including clubmosses such as Baragwanathia; most were under 10 centimetres (3.9 in) high, and some appear closely related to vascular plants, the group that includes trees.[105]
By the Late Devonian, trees such as Archaeopteris were so abundant that they changed river systems from mostly braided to mostly meandering, because their roots bound the soil firmly.[106] In fact they caused a "Late Devonian wood crisis",[107] because:
- They removed more carbon dioxide from the atmosphere, reducing the greenhouse effect and thus causing an ice age in the Carboniferous period.[108] In later ecosystems the carbon dioxide "locked up" in wood is returned to the atmosphere by decomposition of dead wood. However the earliest fossil evidence of fungi that can decompose wood also comes from the Late Devonian.[109]
- The increasing depth of plants' roots led to more washing of nutrients into rivers and seas by rain. This caused algal blooms whose high consumption of oxygen caused anoxic events in deeper waters, increasing the extinction rate among deep-water animals.[108]
Land invertebrates
Animals had to change their feeding and excretory systems, and most land animals developed internal fertilization of their eggs. The difference in refractive index between water and air required changes in their eyes. On the other hand in some ways movement and breathing became easier, and the better transmission of high-frequency sounds in air encouraged the development of hearing.[104]
Some trace fossils from the Cambrian-Ordovician boundary about are interpreted as the tracks of large amphibious arthropods on coastal sand dunes, and may have been made by euthycarcinoids,[110] which are thought to be evolutionary "aunts" of myriapods.[111] Other trace fossils from the Late Ordovician a little over probably represent land invertebrates, and there is clear evidence of numerous arthropods on coasts and alluvial plains shortly before the Silurian-Devonian boundary, about , including signs that some arthropods ate plants.[112] Arthropods were well pre-adapted to colonise land, because their existing jointed exoskeletons provided protection against desiccation, support against gravity and a means of locomotion that was not dependent on water.[113]
The fossil record of other major invertebrate groups on land is poor: none at all for non-parasitic flatworms, nematodes or nemerteans; some parasitic nematodes have been fossilized in amber; annelid worm fossils are known from the Carboniferous, but they may still have been aquatic animals; the earliest fossils of gastropods on land date from the Late Carboniferous, and this group may have had to wait until leaf litter became abundant enough to provide the moist conditions they need.[104]
Land vertebrates

| "Fish" |
|
||||||||||||||||||||||||||||||||||||||||||||||||
Tetrapods, vertebrates with four limbs, evolved from the most closely-related fish in a relatively short timespan in the Late Devonian, from to .[115] From the 1950s to the early 1980s it was thought that tetrapods evolved from fish that had already acquired the ability to crawl on land, possibly in order to go from a pool that was drying out to one that was deeper. However in 1987 nearly-complete fossils of Acanthostega from about showed that this Late Devonian transitional animal had legs and both lungs and gills, but could never have survived on land: its limbs and its wrist and ankle joints were too weak to bear its weight; its ribs were too short to prevent its lungs from being squeezed flat by its weight; its fish-like tail fin would have been damaged by dragging on the ground. The current hypothesis is that Acanthostega, which was about 1 metre (3.3 ft) long, was a wholly aquatic predator that hunted in shallow water. Its skeleton differed from that of most fish, in ways that enabled it to raise its head to breathe air while its body remained submerged, including: its jaws show modifications that would have enabled it to gulp air; the bones at the back of its skull are locked together, providing strong attachment points for muscles that raised its head; the head is not joined to the shoulder girdle and it has a distinct neck.[116]
The Devonian proliferation of land plants may help to explain why air-breathing would have been an advantage: leaves falling into streams and rivers would have encouraged the growth of aquatic vegetation; this would have attracted grazing invertebrates and small fish that preyed on them; they would have been attractive prey but the environment was unsuitable for the big marine predatory fish; air-breathing would have been necessary because these waters would have been short of oxygen, since warm water holds less dissolved oxygen than cooler marine water and since the decomposition of vegetation would have used some of the oxygen.[116]
Later discoveries revealed earlier transitional forms between Acanthostega and completely fish-like animals.[117] Unfortunately there is then a gap of about 30 million years between the fossils of ancestral tetrapods and Mid Carboniferous fossils of vertebrates that look well-adapted for life on land. Some of these look like early relatives of modern amphibians, most of which need to keep their skins moist and to lay their eggs in water, while others are accepted as early relatives of the amniotes, whose water-proof skins and eggs enable them to live and breed far from water.[114]
Dinosaurs, birds and mammals
| Amniotes |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Amniotes, whose eggs can survive in dry environments, probably evolved in the Late Carboniferous period, between and . The earliest fossils of the two surviving amniote groups, synapsids and sauropsids, date from around .[119][120] The synapsid pelycosaurs and their descendants the therapsids are the most common land vertebrates in the best-known Permian fossil beds, between and . However at the time these were all in temperate zones at middle latitudes, and there is evidence that hotter, drier environments nearer the Equator were dominated by sauropsids and amphibians.[121]
The Permian-Triassic extinction wiped out almost all land vertebrates,[122] as well as the great majority of other life.[123] During the slow recovery from this catastrophe, a previously obscure group became the most abundant and diverse terrestrial vertebrates: a few fossils of archosauriformes ("shaped like archosaurs") have been found in Late Permian rocks,[124] but by the Mid Triassic archosaurs were the dominant land vertebrates. Dinosaurs evolved from one group of archosaurs in the Late Triassic, and became the dominant land vertebrates of the Jurassic and Cretaceous periods, between and .[125]
In the Late Jurassic birds evolved from one group of small, predatory theropod dinosaurs. The first birds inherited teeth and long, bony tails from their dinosaur ancestors,[126] but some developed horny, toothless beaks by the very Late Jurassic[127] and short pygostyle tails by the Early Cretaceous.[128]
While the archosaurs and dinosaurs were becoming more dominant in the Triassic, the mammaliform successors of the therapsids could only survive as small, mainly nocturnal insectivores. This apparent set-back may actually have promoted the evolution of mammals, for example nocturnal life may have have accelerated the development of endothermy ("warm-bloodedness") and hair or fur.[129] By in the Early Jurassic there were animals that were very nearly mammals.[130] Unfortunately there is a gap in the fossil record throughout the Mid Jurassic.[131] However fossil teeth discovered in Madagascar indicate that true mammals existed at least .[132] After dominating land vertebrate niches for about 150 million years, the dinosaurs perished in the Cretaceous–Tertiary extinction along with many other groups of organisms.[133] Mammals throughout the time of the dinosaurs had been restricted to a narrow range of taxa, sizes and shapes, but increased rapidly in size and diversity after the extinction,[134][135] with bats taking to the air within 13 million years,[136] and whales to the sea within 15 million years.[137]
Flowering plants
|
|
The 250,000 to 400,000 species of flowering plants outnumber all other ground plants combined, and are the dominant vegetation in most terrestrial ecosystems. There is fossil evidence that flowering plants diversified rapidly in the Early Cretaceous, between and ,[138][139] and that their rise was associated with that of pollinating insects.[139] Among modern flowering plants Magnolias are thought to be close to the common ancestor of the group.[138] However paleontologists have not succeeded in identifying the earliest stages in the evolution of flowering plants.[138][139]
Social insects
The social insects are remarkable because the great majority of individuals in each colony are sterile. This appears contrary to basic concepts of evolution such as natural selection and the selfish gene. In fact there are very few eusocial insect species: only 15 out of approximately 2,600 living families of insects contain eusocial species, and it seems that eusociality has evolved independently only 12 times among arthropods, although some eusocial lineages have diversified into several families. Nevertheless social insects have been spectacularly successful; for example although ants and termites account for only about 2% of known insect species, they form over 50% of the total mass of insects. Their ability to control a territory appears to be the foundation of their success.[140]

The sacrifice of breeding opportunities by most individuals has long been explained as a consequence of these species' unusual haplodiploid method of sex determination, which has the paradoxical consequence that two sterile worker daughters of the same queen share more genes with each other than they would with their offspring if they could breed.[141] However Wilson and Hölldobler argue that this explanation is faulty: for example, it is based on kin selection, but there is no evidence of nepotism in colonies that have multiple queens. Instead, they write, eusociality evolves only in species that are under strong pressure from predators and competitors, but in environments where it is possible to build "fortresses"; after colonies have established this security, they gain other advantages though co-operative foraging. In support of this explanation they cite the appearance of eusociality in bathyergid mole rats,[140] which are not haplodiploid.[142]
The earliest fossils of insects have been found in Early Devonian rocks from about , which preserve only a few varieties of flightless insect. The Mazon Creek lagerstätten from the Late Carboniferous, about , include about 200 species, some gigantic by modern standards, and indicate that insects had occupied their main modern ecological niches as herbivores, detritivores and insectivores. Social termites and ants first appear in the Early Cretaceous, and advanced social bees have been found in Late Cretaceous rocks but did not become abundant until the Mid Cenozoic.[143]
Humans
Modern humans evolved from a lineage of upright-walking apes that has been traced back over to Sahelanthropus.[144] The first known stone tools were made about , apparently by Australopithecus garhi, and were found near animal bones that bear scratches made by these tools.[145] The earliest hominines had chimp-sized brains, but there has been a four-fold increase in the last 3 million years; a statistical analysis suggests that hominine brain sizes depend almost completely on the date of the fossils, while the species to which they are assigned has only slight influence.[146] There is a long-running debate about whether modern humans evolved all over the world from existing advanced hominines or are descendants of a single small population in Africa, which then migrated all over the world less than 200,000 years ago and replaced previous hominine species.[147] There is also debate about whether anatomically-modern humans had an intellectual, cultural and technological "Great Leap Forward" under 100,000 years ago and, if so, whether this was due to neurological changes that are not visible in fossils.[148]
Mass extinctions
Life on earth has suffered occasional mass extinctions at least since . Although they are disasters at the time, mass extinctions have sometimes accelerated the evolution of life on earth. When dominance of particular ecological niches passes from one group of organisms to another, it is rarely because the new dominant group is "superior" to the old and usually because an extinction event eliminates the old dominant group and makes way for the new one.[149][150]
The fossil record appears to show that the gaps between mass extinctions are becoming longer and the average and background rates of extinction are decreasing. Both of these phenomena could be explained in one or more ways:[151]
- The oceans may have become more hospitable to life over the last 500 million years and less vulnerable to mass extinctions: dissolved oxygen became more widespread and penetrated to greater depths; the development of life on land reduced the run-off of nutrients and hence the risk of eutrophication and anoxic events; and marine ecosystems became more diversified so that food chains were less likely to be disrupted.[152][153]
- Reasonably complete fossils are very rare, most extinct organisms are represented only by partial fossils, and complete fossils are rarest in the oldest rocks. So paleontologists have mistakenly assigned parts of the same organism to different genera which were often defined solely to accommodate these finds – the story of Anomalocaris is an example of this. The risk of this mistake is higher for older fossils because these are often unlike parts of any living organism. Many of the "superfluous" genera are represented by fragments which are not found again and the "superfluous" genera appear to become extinct very quickly.[151]
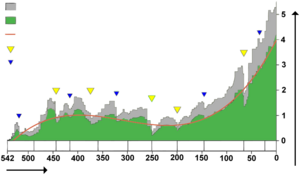
Biodiversity in the fossil record, which is
-
- "the number of distinct genera alive at any given time; that is, those whose first occurrence predates and whose last occurrence postdates that time"[154]
shows a different trend: a fairly swift rise from ; a slight decline from , in which the devastating Permian–Triassic extinction event is an important factor; and a swift rise from to the present.[154]
The present
Oxygenic photosynthesis accounts for virtually all of the production of organic matter from non-organic ingredients. Production is split about evenly between land and marine plants, and phytoplankton are the dominant marine producers.[155]
The processes that drive evolution are still operating. Well-known examples include the changes in coloration of the peppered moth over the last 200 years and the more recent appearance of pathogens that are resistant to antibiotics.[156][157] There is even evidence that humans are still evolving, and possibly at an accelerating rate over the last 40,000 years.[158]
See also
|
|||||||||||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||||||||
Footnotes
- ↑ Name given as in Butterfield's paper "Bangiomorpha pubescens ..." (2000). A fossil fish, also from China, has also been named Qingshania. The name of one of these will have to change.
- ↑ Myxozoa were thought to be an exception, but are now thought to be heavily modified members of the Cnidaria: Jímenez-Guri, E., Philippe, H., Okamura, B., and Holland, P.W.H. (July 2007). "Buddenbrockia is a cnidarian worm". Science 317 (116): 116–118. doi:. PMID 17615357. http://www.sciencemag.org/cgi/content/abstract/317/5834/116. Retrieved on 2008-09-03.
References
- ↑ 1.0 1.1
- Dalrymple, G.B. (1991). The Age of the Earth. California: Stanford University Press. ISBN 0-8047-1569-6.
- Newman, William L. (2007-07-09). "Age of the Earth". Publications Services, USGS. Retrieved on 2008-08-29.
- Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". Geological Society, London, Special Publications 190: 205–221. doi:. http://sp.lyellcollection.org/cgi/content/abstract/190/1/205. Retrieved on 2007-09-20.
- ↑ E. M. Galimov and A. M. Krivtsov (December 2005). "Origin of the Earth-Moon System". J. Earth Syst. Sci. 114 (6): 593–600. doi:. [1]
- ↑ 3.0 3.1 Cohen, B.A., Swindle, T.D., and Kring, D.A. (December 2000). "Support for the Lunar Cataclysm Hypothesis from Lunar Meteorite Impact Melt Ages". Science 290 (5497): 1754–1756. doi:. PMID 11099411. http://www.sciencemag.org/cgi/content/abstract/290/5497/1754. Retrieved on 2008-08-31.
- ↑
- "Early Earth Likely Had Continents And Was Habitable" (2005-11-17).
- Cavosie, A. J.; J. W. Valley, S. A., Wilde, and E.I.M.F. (July 15, 2005). "Magmatic δ18O in 4400-3900 Ma detrital zircons: A record of the alteration and recycling of crust in the Early Archean". Earth and Planetary Science Letters 235 (3-4): 663–681. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6V61-4GDKB05-3&_coverDate=07%2F15%2F2005&_alid=382434001&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=5801&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=be47e49c535d059be188b66c6e596dd5.
- ↑ Britt, Robert Roy (2002-07-24). "Evidence for Ancient Bombardment of Earth". Space.com. Retrieved on 2006-04-15.
- ↑ Valley, J.W., Peck, W.H., King, E.M., and Wilde, S.A. (April 2002). "A cool early Earth" (PDF). Geology 30 (4): 351–354. doi:. http://www.geology.wisc.edu/zircon/Valley2002Cool_Early_Earth.pdf. Retrieved on 2008-09-13.
- ↑ Dauphas, N., Robert, F., and Marty, B. (December 2000). "The Late Asteroidal and Cometary Bombardment of Earth as Recorded in Water Deuterium to Protium Ratio". Icarus 148 (2): 508–512. doi:.
- ↑ 8.0 8.1 Brasier, M., McLoughlin, N., Green, O., and Wacey, D. (June 2006). "A fresh look at the fossil evidence for early Archaean cellular life" (PDF). Philosophical Transactions of the Royal Society: Biology 361 (1470): 887–902. doi:. http://physwww.mcmaster.ca/~higgsp/3D03/BrasierArchaeanFossils.pdf. Retrieved on 2008-08-30.
- ↑
- Schopf, J.W. (April 1993). "Microfossils of the Early Archean Apex Chert: New Evidence of the Antiquity of Life". Science 260 (5108): 640–646. doi:. PMID 11539831. http://www.sciencemag.org/cgi/content/abstract/260/5108/640. Retrieved on 2008-08-30.
- Altermann, W.; J. Kazmierczak (2003). "Archean microfossils: a reappraisal of early life on Earth". Res Microbiol 154 (9): 611–7. doi:. PMID 14596897.
- ↑ Mojzsis, S.J., Arrhenius, G., McKeegan, K.D.,Harrison, T.M., Nutman, A.P., and Friend, C.R.L. (November 1996). "Evidence for life on Earth before 3,800 million years ago". Nature 384: 55–59. doi:. http://www.nature.com/nature/journal/v384/n6604/abs/384055a0.html. Retrieved on 2008-08-30.
- ↑ 11.0 11.1 Grotzinger, J.P., and Rothman, D.H. (1996). "An abiotic model for stomatolite morphogenesis". Nature 383: 423–425. doi:.
- ↑
- Fedo, C.M., and Whitehouse, M.J. (May 2002). "Metasomatic Origin of Quartz-Pyroxene Rock, Akilia, Greenland, and Implications for Earth's Earliest Life". Science 296 (5572): 1448–1452. doi:. PMID 12029129. http://www.sciencemag.org/cgi/content/abstract/296/5572/1448. Retrieved on 2008-08-30.
- Lepland, A., van Zuilen, M.A., Arrhenius, G., Whitehouse M.J., and Fedo, C.M. (January 2005). "Questioning the evidence for Earth's earliest life — Akilia revisited". Geology 33 (1): 77–79. doi:. http://geology.geoscienceworld.org/cgi/content/abstract/33/1/77. Retrieved on 2008-08-30.
- ↑ Schopf, J. (2006). "Fossil evidence of Archaean life". Philos Trans R Soc Lond B Biol Sci 361 (1470): 869–85. doi:. PMID 16754604.
- ↑ Ciccarelli, F.D.; T. Doerks, C. von Mering, C.J. Creevey, et al. (2006). "Toward automatic reconstruction of a highly resolved tree of life". Science 311 (5765): 1283–7. doi:. PMID 16513982.
- ↑ Mason, S.F. (1984). "Origins of biomolecular handedness". Nature 311 (5981): 19–23. doi:. PMID 6472461.
- ↑ Orgel, L.E. (October 1994). "The origin of life on the earth" (PDF). Scientific American 271 (4): 76–83. http://courses.washington.edu/biol354/The%20Origin%20of%20Life%20on%20Earth.pdf. Retrieved on 2008-08-30. Also available as a web page }}
- ↑ 17.0 17.1 17.2 Cowen, R.. History of Life (3rd ed.). Blackwell Science. p. p. 6. ISBN 0632044446.
- ↑ O'Leary, M.R. (2008). Anaxagoras and the Origin of Panspermia Theory. iUniverse, Inc.. ISBN 0595495966.
- ↑ 19.0 19.1 Arrhenius, S. (1903). "The Propagation of Life in Space". Die Umschau volume=7. Reprinted in Goldsmith, D.,, ed.. The Quest for Extraterrestrial Life. University Science Books. ISBN 0198557043.
- ↑ Hoyle, F., and Wickramasinghe, C. (1979). "On the Nature of Interstellar Grains". Astrophysics and Space Science 66: 77–90. doi:.
- ↑ 21.0 21.1 Crick, F. H.; Orgel, L. E. (1973), "Directed Panspermia", Icarus 19: 341–348, doi:
- ↑ 22.0 22.1 22.2 22.3 Warmflash, D.; B. Weiss (November 2005). "Did Life Come From Another World?". Scientific American: 64–71. http://www.sciam.com/article.cfm?articleID=00073A97-5745-1359-94FF83414B7F0000&pageNumber=1&catID=2. Retrieved on 2008-09-02.
- ↑ "Claim of Martian Life Called 'Bogus'" (August 2007). Retrieved on 2008-09-02.
- ↑ Peretó, J. (2005). "Controversies on the origin of life" (PDF). Int. Microbiol. 8 (1): 23–31. PMID 15906258. http://www.im.microbios.org/0801/0801023.pdf. Retrieved on 2007-10-07.
- ↑ Szathmáry, E. (February 2005). "Life: In search of the simplest cell". Nature 433: 469–470. doi:. http://www.nature.com/nature/journal/v433/n7025/full/433469a.html. Retrieved on 2008-09-01.
- ↑ Luisi, P.L.; F. Ferri, P. Stano (2006). "Approaches to semi-synthetic minimal cells: a review". Naturwissenschaften 93 (1): 1–13. doi:. PMID 16292523.
- ↑ Joyce, G.F. (2002). "The antiquity of RNA-based evolution". Nature 418 (6894): 214–21. doi:. PMID 12110897.
- ↑ 28.0 28.1 Hoenigsberg, H.. "Evolution without speciation but with selection: LUCA, the Last Universal Common Ancestor in Gilbert’s RNA world". Genetic and Molecular Research 2 (4): 366–375. PMID 15011140. http://www.funpecrp.com.br/gmr/year2003/vol4-2/gmr0070_full_text.htm. Retrieved on 2008-08-30.(also available as PDF
- ↑ Trevors, J.T.; D.L. Abel (2004). "Chance and necessity do not explain the origin of life". Cell Biol. Int. 28 (11): 729–39. doi:. PMID 15563395.
- ↑ Forterre, P.; N. Benachenhou-Lahfa, F. Confalonieri, M. Duguet, et al. (1992). "The nature of the last universal ancestor and the root of the tree of life, still open questions". BioSystems 28 (1–3): 15–32. doi:. PMID 1337989.
- ↑ Cech, T.R. (August 2000). "The ribosome is a ribozyme". Science 289 (5481): 878–9. doi:. PMID 10960319. http://www.sciencemag.org/cgi/content/short/289/5481/878. Retrieved on 2008-09-01.
- ↑ Johnston, W. K.; et al. (2001). "RNA-Catalyzed RNA Polymerization: Accurate and General RNA-Templated Primer Extension". Science 292 (5520): 1319–1325. doi:. PMID 11358999.
- ↑ 33.0 33.1
- Levy, Matthew; Miller, Stanley L. (1998). "The stability of the RNA bases: Implications for the origin of life". PNAS 95: 7933–7938. doi:. PMID 9653118.
- Larralde, R.; Robertson, M. P.; Miller, S. L. (1995). "Rates of Decomposition of Ribose and Other Sugars: Implications for Chemical Evolution". PNAS 92 (18): 8158–8160. doi:. PMID 7667262.
- Lindahl, Tomas (1993). "Instability and decay of the primary structure of DNA". Nature 362 (6422): 709–715. doi:.
- ↑ Orgel, Leslie (November 2000). "A Simpler Nucleic Acid". Science 290 (5495): 1306–7. doi:. PMID 11185405.
- ↑ Nelson, K.E.; Levy, M.; Miller, S.L. (April 2000). "Peptide nucleic acids rather than RNA may have been the first genetic molecule". Proceedings of the National Academy of Sciences 97 (8): 3868–71. doi:. PMID 10760258.
- ↑ Martin, W. and Russell, M.J. (2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society: Biological 358: 59–85. doi:. PMID 12594918. http://www.ncbi.nlm.nih.gov/pubmed/12594918. Retrieved on 2008-09-05.
- ↑ Günter Wächtershäuser (August 2000). "Life as We Don't Know It". Science 289: 1307–1308. doi:. PMID 10979855.
- ↑ Trevors, J.T.; R. Psenner (2001). "From self-assembly of life to present-day bacteria: a possible role for nanocells". FEMS Microbiol. Rev. 25 (5): 573–82. doi:. PMID 11742692.
- ↑ Segré, D., Ben-Eli, D., Deamer, D., and Lancet, D. (February-April 2001). "The Lipid World" (PDF). Origins of Life and Evolution of Biospheres 2001 31 (1-2): 119–45. doi:. PMID 11296516. http://ool.weizmann.ac.il/Segre_Lipid_World.pdf. Retrieved on 2008-09-01.
- ↑ Cairns-Smith, A.G. (1968), "An approach to a blueprint for a primitive organism", in Waddington, C,H., Towards a Theoretical Biology, 1, Edinburgh University Press, pp. 57–66
- ↑ Ferris, J.P. (June 1999). "Prebiotic Synthesis on Minerals: Bridging the Prebiotic and RNA Worlds". Biological Bulletin. Evolution: A Molecular Point of View 196 (3): 311. doi:. http://www.jstor.org/pss/1542957. Retrieved on 2008-09-01.
- ↑ Hanczyc, M.M., Fujikawa, S.M. and Jack W. Szostak, J.W. (October 2003). "Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division". Science 302 (5645): 618–622. doi:. PMID 14576428. http://www.sciencemag.org/cgi/content/abstract/302/5645/618. Retrieved on 2008-09-01.
- ↑ Hartman, H. (October 1998). "Photosynthesis and the Origin of Life". Origins of Life and Evolution of Biospheres 28 (4–6): 512–521. http://www.springerlink.com/content/t1n325268n01217k/. Retrieved on 2008-09-01.
- ↑ 44.0 44.1 Krumbein, W.E., Brehm, U., Gerdes, G., Gorbushina, A.A., Levit, G. and Palinska, K.A. (2003), "Biofilm, Biodictyon, Biomat Microbialites, Oolites, Stromatolites, Geophysiology, Global Mechanism, Parahistology", in Krumbein, W.E., Paterson, D.M., and Zavarzin, G.A. (PDF), Fossil and Recent Biofilms: A Natural History of Life on Earth, Kluwer Academic, pp. 1–28, ISBN 1402015976, http://134.106.242.33/krumbein/htdocs/Archive/397/Krumbein_397.pdf, retrieved on 2008-07-09
- ↑ Risatti, J,B., Capman, W.C., and Stahl, D.A. (October 11, 1994). "Community structure of a microbial mat: the phylogenetic dimension" (PDF). Proceedings of the National Academy of Sciences 91 (21): 10173–10177. doi:. PMID 7937858. http://www.pnas.org/content/91/21/10173.full.pdf. Retrieved on 2008-07-09.
- ↑ Allwood, A.C., Walter, M.R., Kamber, B.S., Marshall1, C.P., and Burch, I.W. (June 2006)). "Stromatolite reef from the Early Archaean era of Australia". Nature 441: 714–718. doi:. http://www.nature.com/nature/journal/v441/n7094/abs/nature04764.html. Retrieved on 2008-08-31.
- ↑ Blankenship, R.E. (1 January 2001). "Molecular evidence for the evolution of photosynthesis". Trends in Plant Science 6 (1): 4–6. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TD1-424KK4J-3&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=6f38f9f1d29b24fc90d0145837338b9e. Retrieved on 2008-07-14.
- ↑ Hoehler, T.M., Bebout, B.M., and Des Marais, D.J. (19 July 2001). "The role of microbial mats in the production of reduced gases on the early Earth". Nature 412: 324–327. doi:. http://www.nature.com/nature/journal/v412/n6844/full/412324a0.html. Retrieved on 2008-07-14.
- ↑ Abele, D. (7 November 2002). "Toxic oxygen: The radical life-giver". Nature 420 (27): 27. doi:. http://www.nature.com/nature/journal/v420/n6911/full/420027a.html. Retrieved on 2008-07-14.
- ↑ "Introduction to Aerobic Respiration". University of California, Davis. Retrieved on 2008-07-14.
- ↑ Goldblatt, C., Lenton, T.M., and Watson, A.J. (2006). "The Great Oxidation at ~2.4 Ga as a bistability in atmospheric oxygen due to UV shielding by ozone" (PDF). Geophysical Research Abstracts 8 (00770). http://www.cosis.net/abstracts/EGU06/00770/EGU06-J-00770.pdf. Retrieved on 2008-09-01.
- ↑ 52.0 52.1 Glansdorff, N., Xu, Y., and Labedan, B. (2008). "The Last Universal Common Ancestor: emergence, constitution and genetic legacy of an elusive forerunner". Biology Direct 3 (29): 29. doi:.
- ↑ 53.0 53.1 Brocks, J.J., Logan, G.A., Buick, R., and Summons, R.E. (1999). "Archaean molecular fossils and the rise of eukaryotes". Science 285: 1033–1036. doi:. PMID 10446042. http://www.sciencemag.org/cgi/content/abstract/285/5430/1033. Retrieved on 2008-09-02.
- ↑ 54.0 54.1 Hedges, S.B., Blair, J.E, Venturi, M.L., and Shoe, J.L (January 2004). "A molecular timescale of eukaryote evolution and the rise of complex multicellular life". BMC Evolutionary Biology 4 (2): 2. doi:. http://www.biomedcentral.com/1471-2148/4/2/abstract/. Retrieved on 2008-07-14.
- ↑ 55.0 55.1 Nisbet, E.G., and Fowler, C.M.R. (December 7 1999). "Archaean metabolic evolution of microbial mats". Proceedings of the Royal Society: Biology 266 (1436): p. 2375. doi:. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1690475. Retrieved on 2008-07-16. - abstract with link to free full content (PDF)
- ↑ Burki, F., Shalchian-Tabrizi, K., Minge, M., Skjæveland, Å., Nikolaev, S.I., et al. (2007). "Phylogenomics Reshuffles the Eukaryotic Supergroups". PLoS ONE 2 (8: e790): e790. doi:.
- ↑ Parfrey, L.W., Barbero, E., Lasser, E., Dunthorn, M., Bhattacharya, D., Patterson, D.J., and Katz, L.A. (December 2006). "Evaluating Support for the Current Classification of Eukaryotic Diversity". PLoS Genetics 2 (12): e220. doi:. PMID 17194223. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1713255.
- ↑ Margulis, L. (1981). Symbiosis in cell evolution. San Francisco: W.H. Freeman. ISBN 0716712563.
- ↑ Vellai, T. and Vida, G. (1999). "The origin of eukaryotes; the difference between eukaryotic and prokaryotic cells". Proceedings of the Royal Society: Biology 266: 1571–1577. doi:.
- ↑ Selosse, M-A., Abert, B., and Godelle, B. (2001). "Reducing the genome size of organelles favours gene transfer to the nucleus". Trends in ecology & evolution 16 (3): 135–141. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VJ1-429XTFM-H&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=8370ca16bcde45bfa1c050068a2d6e19. Retrieved on 2008-09-02.
- ↑ Pisani, D., Cotton, J.A., McInerney, J.O. (2007). "Supertrees disentangle the chimerical origin of eukaryotic genomes". Mol Biol Evol. 24 (8): 1752–60. doi:. PMID 17504772.
- ↑ Gray, M.W., Burger, G., and Lang, B.F. (1999). "Mitochondrial evolution". Science 283 (5407): 1476–1481. doi:. PMID 10066161. http://www.sciencemag.org/cgi/content/abstract/283/5407/1476. Retrieved on 2008-09-02.
- ↑ Rasmussen, B., Fletcher, I.R., Brocks, J.R., and Kilburn, M.R. (October 2008). "Reassessing the first appearance of eukaryotes and cyanobacteria". Nature 455: 1101-1104. doi:.
- ↑ 64.0 64.1 64.2 64.3 Fedonkin, M.A. (March 2003). "The origin of the Metazoa in the light of the Proterozoic fossil record" (PDF). Paleontological Research 7 (1): 9–41. doi:. http://www.vend.paleo.ru/pub/Fedonkin_2003.pdf. Retrieved on 2008-09-02.
- ↑ Han, T.M. and Runnegar, B. (July 1992). Megascopic eukaryotic algae from the 2.1-billion-year-old negaunee iron-formation, Michigan. pp. 232–235. doi:. PMID 1631544. http://www.sciencemag.org/cgi/content/abstract/257/5067/232. Retrieved on 2008-09-02.
- ↑ Javaux, E.J, Knoll, A.H., and Walter, M.R. (September 2004). "TEM evidence for eukaryotic diversity in mid-Proterozoic oceans". Geobiology 2 (3): 121–132. doi:. http://www3.interscience.wiley.com/journal/118814335/abstract. Retrieved on 2008-09-02.
- ↑ 67.0 67.1 Butterfield, N.J. (2005). "Probable Proterozoic fungi". Paleobiology 31 (1): 165–182. doi:. http://paleobiol.geoscienceworld.org/cgi/content/abstract/31/1/165. Retrieved on 2008-09-02.
- ↑ 68.0 68.1 Nakagaki, T., Yamada H. and Tóth, Á. (September 2000). "Intelligence: Maze-solving by an amoeboid organism". Nature 407: 470. doi:. http://www.nature.com/nature/journal/v407/n6803/abs/407470a0.html. Retrieved on 2008-09-03.
- ↑ Bell, G., and Mooers, A.O. (1968). "Size and complexity among multicellular organisms". Biological Journal of the Linnean Society 60 (3): 345–363. doi:. http://www3.interscience.wiley.com/journal/119168103/abstract. Retrieved on 2008-09-03.
- ↑ Kaiser, D. (2001). "Building a multicellular organism". Annual Review of Genetetics 35: 103–123. doi:. PMID 11700279.
- ↑ Bonner, J.T. (January 1999). "The Origins of Multicellularity". Integrative Biology 1 (1): 27–36. doi:. http://doi.wiley.com/10.1002/(SICI)1520-6602(1998)1:1%3C27::AID-INBI4%3E3.0.CO;2-6. Retrieved on 2008-09-03.
- ↑ 72.0 72.1 72.2 72.3 72.4 Butterfield, N.J. (September 2000). "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology 26 (3): 386–404. doi:. http://paleobiol.geoscienceworld.org/cgi/content/abstract/26/3/386. Retrieved on 2008-09-02.
- ↑ Bernstein, H., Byerly, H., Hopf, F., and Michod, R. (1984). "Origin of sex". Journal of Theoretical Biology 110 (3): 323–351. doi:. PMID 6209512.
- ↑ 74.0 74.1 74.2 Judson, O. (2002). Dr. Tatiana's sex advice to all creation. New York: Metropolitan Books. pp. 233–4. ISBN 0-8050-6331-5.
- ↑ Hickey, D. (1982). "Selfish DNA: a sexually-transmitted nuclear parasite". Genetics 101 (3–4): 519–31. PMID 6293914.
- ↑ DasSarma, S. (2007). "Extreme Microbes". Extreme Microbes. 95. pp. page 224–231.
- ↑ Sterrer, W. (2002). "On the origin of sex as vaccination". Journal of Theoretical Biology 216: 387–96. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WMD-46DM0JD-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=3f5b76ba0b7fff999ee670479f06e5af. Retrieved on 2008-08-02.
- ↑ Holmes R.K., and Jobling, M.G. (1996), "Genetics: Exchange of Genetic Information", in Baron, S., Baron's Medical Microbiology (4th ed.), Galveston: University of Texas Medical Branch, ISBN 0-9631172-1-1, http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=conjugation&rid=mmed.section.468, retrieved on 2008-09-02
- ↑ Christie, P.J. (April 2001). "Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines". Molecular Microbiology 40 (22): 294–305. doi:. http://lib.bioinfo.pl/meid:10183. Retrieved on 2008-09-02.
- ↑ Dong, L., Xiao, S., Shen, B., and Zhou, C. (January 2008). "Silicified Horodyskia and Palaeopascichnus from upper Ediacaran cherts in South China: tentative phylogenetic interpretation and implications for evolutionary stasis". Journal of the Geological Society 165: 367–378. doi:. http://findarticles.com/p/articles/mi_qa3721/is_200801/ai_n24394476/pg_1?tag=artBody;col1. Retrieved on 2008-09-02.
- ↑ Gaidos, E., Dubuc, T., Dunford, M., McAndrew, P., Padilla-gamiño, J., Studer, B., Weersing, K., and Stanley, S. (2007). "The Precambrian emergence of animal life: a geobiological perspective" (PDF). Geobiology 5: 351. doi:. http://www.soest.hawaii.edu/GG/FACULTY/GAIDOS/geobiology2007.pdf. Retrieved on 2008-09-03.
- ↑ >Davidson, M.W.. "Animal Cell Structure" (in English). Florida State University. Retrieved on 2008-09-03.
- ↑ Saupe, S.G. "Concepts of Biology" (in English). College of St. Benedict / St. John's University. Retrieved on 2008-09-03.
- ↑ Hinde, R.T., (1998). "The Cnidaria and Ctenophora". in Anderson, D.T.,. Invertebrate Zoology. Oxford University Press. pp. 28-57. ISBN 0195513681.
- ↑ Chen, J.-Y., Oliveri, P., Gao, F., Dornbos, S.Q., Li, C-W., Bottjer, D.J. and Davidson, E.H. (August 2002). "Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China" (PDF). Developmental Biology 248 (1): 182–196. doi:. http://www.uwm.edu/~sdornbos/PDF's/Chen%20et%20al.%202002.pdf. Retrieved on 2008-09-03.
- ↑ Grazhdankin (2004). "Patterns of distribution in the Ediacaran biotas: facies versus biogeography and evolution". Paleobiology 30: 203. doi:. ISSN 0094–8373.
- ↑ Seilacher, A. (1992). "Vendobionta and Psammocorallia: lost constructions of Precambrian evolution" (abstract). Journal of the Geological Society, London 149 (4): 607–613. doi:. ISSN 0016–7649. http://jgs.lyellcollection.org/cgi/content/abstract/149/4/607. Retrieved on 2007-06-21.
- ↑ Martin, M.W.; Grazhdankin, D.V.; Bowring, S.A.; Evans, D.A.D.; Fedonkin, M.A.; Kirschvink, J.L. (2000-05-05). "Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution" (abstract). Science 288 (5467): 841. doi:. PMID 10797002. http://www.scienceonline.org/cgi/content/abstract/288/5467/841. Retrieved on 2008-07-03.
- ↑ Fedonkin, M.A.; Waggoner, B. (1997). "The late Precambrian fossil Kimberella is a mollusc-like bilaterian organism" (abstract). Nature 388: 868–871. doi:. http://www.nature.com/nature/journal/v388/n6645/abs/388868a0.html. Retrieved on 2008-07-03.
- ↑ Mooi, R. and Bruno, D. (1999). "Evolution within a bizarre phylum: Homologies of the first echinoderms" (PDF). American Zoologist 38: 965–974. http://icb.oxfordjournals.org/cgi/reprint/38/6/965.pdf. Retrieved on 2007-11-24.
- ↑ McMenamin, M.A.S (2003). "Spriggina is a trilobitoid ecdysozoan" (abstract). Abstracts with Programs (Geological Society of America) 35 (6): 105. http://gsa.confex.com/gsa/2003AM/finalprogram/abstract_62056.htm. Retrieved on 2007-11-24.
- ↑ Lin, J-P., Gon, S.M., Gehling, J.G., Babcock, L.E., Zhao, Y-L., Zhang, X-L,, Hu, S-X., Yuan, J-L., Yu, M-Y., and Peng, J. (March 2006). "A Parvancorina-like arthropod from the Cambrian of South China". Historical Biology: A Journal of Paleobiology 18 (1): 33–45. doi:.
- ↑ 93.0 93.1 Butterfield, N.J. (2006). "Hooking some stem-group "worms": fossil lophotrochozoans in the Burgess Shale". Bioessays 28 (12): 1161–6. doi:.
- ↑ 94.0 94.1 94.2 Bengtson, S. (2004), Early skeletal fossils, in Lipps, J.H., and Waggoner, B.M., "Neoproterozoic - Cambrian Biological Revolutions" (PDF), Palentological Society Papers 10: 67–78, http://www.cosmonova.org/download/18.4e32c81078a8d9249800021554/Bengtson2004ESF.pdf, retrieved on 2008-07-18
- ↑ Gould, S.J. (1989). Wonderful Life: The Burgess Shale and the Nature of History. W.W. Norton & Company.
- ↑ Budd, G.E. (2003). "The Cambrian Fossil Record and the Origin of the Phyla" (Free full text). Integrative and Comparative Biology 43 (1): 157–165. doi:. http://intl-icb.oxfordjournals.org/cgi/content/abstract/43/1/157. Retrieved on 2008-07-15.
- ↑ Budd, G.E. (1996). "The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group". Lethaia 29 (1): 1–14. doi:.
- ↑ Marshall, C.R. (2006). "Explaining the Cambrian “Explosion” of Animals". Annu. Rev. Earth Planet. Sci. 34: 355–384. doi:. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.earth.33.031504.103001?journalCode=earth. Retrieved on 2007-11-06.
- ↑ Conway Morris, S. (August 2, 2003). "Once we were worms". New Scientist 179 (2406): p. 34. http://cas.bellarmine.edu/tietjen/Evolution/once_we_were_worms.htm. Retrieved on 2008-09-05.
- ↑ Shu, D-G., Luo, H-L., Conway Morris, S., Zhang, X-L., Hu, S-X., Chen, L., J. Han, J., Zhu, M., Li, Y., and Chen, L-Z. (November 1999). "Lower Cambrian vertebrates from south China" (PDF). Nature 402: 42–46. doi:. http://www.bios.niu.edu/davis/bios458/Shu1.pdf. Retrieved on 2008-09-05.
- ↑ Shu, D.-G., Conway Morris, S., Han, J., Zhang, Z.-F., Yasui, K., Janvier, P., Chen, L., Zhang, X.-L., Liu, J.-N., Li, Y., and Liu, H.-Q. (January 2003). "Head and backbone of the Early Cambrian vertebrate Haikouichthys". Nature 421: 526–529. doi:. http://www.nature.com/nature/journal/v421/n6922/abs/nature01264.html. Retrieved on 2008-09-05.
- ↑ Sansom I.J., Smith, M.M., and Smith, M.P. (2001), "The Ordovician radiation of vertebrates", in Ahlberg, P.E., Major Events in Early Vertebrate Evolution, Taylor and Francis, pp. 156–171, ISBN 0-415-23370-4
- ↑ 103.0 103.1 Cowen, R.. History of Life (3rd ed.). Blackwell Science. pp. 120-122. ISBN 0632044446.
- ↑ 104.0 104.1 104.2 Selden, P.A. (2001), ""Terrestrialization of Animals"", in Briggs, D.E.G., and Crowther, P.R., Palaeobiology II: A Synthesis, Blackwell, pp. 71–74, ISBN 0632051493, http://books.google.co.uk/books?id=AHsrhGOTRM4C&pg=PA71&lpg=PA71&dq=%22Terrestrialization+of+Animals%22+selden&source=web&ots=ImrDW71qDp&sig=JptdVx34SMIjKamHXDnEpKSE78s&hl=en&sa=X&oi=book_result&resnum=1&ct=result#PPA74,M1, retrieved on 2008-09-05
- ↑ 105.0 105.1 Kenrick, P., and Crane, P.R. (September 1997). "The origin and early evolution of plants on land" (PDF). Nature 389: 33. doi:. http://botit.botany.wisc.edu/courses/botany_940/06EvidEvol/papers/KendrickCrane1997.pdf. Retrieved on 2008-09-05.
- ↑ Scheckler, S.E. (2001), ""Afforestation – the First Forests"", in Briggs, D.E.G., and Crowther, P.R., Palaeobiology II: A Synthesis, Blackwell, pp. 67–70, ISBN 0632051493, http://books.google.co.uk/books?id=AHsrhGOTRM4C&pg=PA69&lpg=PA69&dq=devonian+meandering+plants+trees&source=web&ots=ImrDW61pBt&sig=RsDgJXv-NNu6Rxk_yFpb5espyLY&hl=en&sa=X&oi=book_result&resnum=3&ct=result, retrieved on 2008-09-05
- ↑ The phrase "Late Devonian wood crisis" is used at "Palaeos – Tetrapoda: Acanthostega". Retrieved on 2008-09-05.
- ↑ 108.0 108.1 Algeo, T.J., and Scheckler, S.E. (1998). "Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events". Philosophical Transactions of the Royal Society: Biology 353: 113–130. doi:. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1692181&blobtype=pdf. Retrieved on 2008-09-05.
- ↑ Taylor T.N., and Osborn J.M. (1996). "The importance of fungi in shaping the paleoecosystem". Review of Paleobotany and Palynology 90: 249–262. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6V6W-454YDFK-7&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=9d5d008d99d044684e947ad74b05514d. Retrieved on 2008-09-05.
- ↑ MacNaughton, R.B., Cole, J.M., Dalrymple, R.W., Braddy, S.J., Briggs, D.E.G., and Lukie, T.D. (May 2002). "First steps on land: Arthropod trackways in Cambrian-Ordovician eolian sandstone, southeastern Ontario, Canada". Geology 30 (5): 391–394. doi:. http://geology.geoscienceworld.org/cgi/content/abstract/30/5/391. Retrieved on 2008-09-05.
- ↑ Vaccari, N.E., Edgecombe, G.D., Escudero, C. (2004). "Cambrian origins and affinities of an enigmatic fossil group of arthropods". Nature 430: 554–557. doi:.
- ↑ Buatois, L.A., Mangano, M.G., Genise, JF., and Taylor, T.N. (June 1998). "The ichnologic record of the continental invertebrate invasion; evolutionary trends in environmental expansion, ecospace utilization, and behavioral complexity". Palaios 13 (3): 217–240. doi:. http://palaios.sepmonline.org/cgi/content/abstract/13/3/217. Retrieved on 2008-09-05.
- ↑ Cowen, R.. History of Life (3rd ed.). Blackwell Science. p. p. 126. ISBN 0632044446.
- ↑ 114.0 114.1 Ahlberg, P.E., and Milner, A.R. (April 1994). "The Origin and Early Diversification of Tetrapods". Nature 368: 507–514. doi:. http://www.nature.com/nature/journal/v368/n6471/abs/368507a0.html. Retrieved on 2008-09-06.
- ↑ Gordon, M.S, Graham, J.B., and Wang, T. (September/October 2004). "Revisiting the Vertebrate Invasion of the Land". Physiological and Biochemical Zoology 77 (5): 697–699. doi:.
- ↑ 116.0 116.1 Clack, J.A. (November, 2005). "Getting a Leg Up on Land". Retrieved on 2008-09-06.
- ↑ Daeschler, E.B., Shubin, N.H., and Jenkins, F.A. (April 2006). "A Devonian tetrapod-like fish and the evolution of the tetrapod body plan" (PDF). Nature 440: 757–763. doi:. http://www.com.univ-mrs.fr/~boudouresque/Publications_DOM_2006_2007/Daeschler_et_al_2006_Nature.pdf. Retrieved on 2008-09-06.
- ↑ Debraga, M., and Rieppel, O. (July 1997). "Reptile phylogeny and the interrelationships of turtles". Zoological Journal of the Linnean Society 120 (3): 281–354. doi:. http://www3.interscience.wiley.com/journal/119830935/abstract. Retrieved on 2008-09-07.
- ↑ 119.0 119.1 Benton M.J., and Donoghue, P.C.J. (2007). "Paleontological Evidence to Date the Tree of Life". Molecular Biology and Evolution 24 (1): 26–53. doi:. PMID 17047029. http://mbe.oxfordjournals.org/cgi/content/full/24/1/26. Retrieved on 2008-09-07.
- ↑ 120.0 120.1 Benton M.J. (May 1990). "Phylogeny of the Major Tetrapod Groups: Morphological Data and Divergence Dates". Journal of Molecular Evolution 30 (5): 409–424. doi:. http://www.springerlink.com/content/k152294003652458/. Retrieved on 2008-09-07.
- ↑ Sidor, C.A., O'Keefe, F.R., Damiani, R., Steyer, J.S., Smith, R.M.H., Larsson, H.C.E., Sereno, P.C., Ide, O, and Maga, A. (April 2005). "Permian tetrapods from the Sahara show climate-controlled endemism in Pangaea". Nature 434: 886–889. doi:. http://www.nature.com/nature/journal/v434/n7035/full/nature03393.html. Retrieved on 2008-09-08.
- ↑ Smith, R., and Botha, J. (September-October 2005). "The recovery of terrestrial vertebrate diversity in the South African Karoo Basin after the end-Permian extinction". Comptes Rendus Palevol 4: 623–636. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6X1G-4GYH7VN-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=add24b0622f2aff0b41e7c42a3160fa7. Retrieved on 2008-09-08.
- ↑ Benton M.J. (2005). When Life Nearly Died: The Greatest Mass Extinction of All Time. Thames & Hudson. ISBN 978-0500285732.
- ↑ Gauthier, J., Cannatella, D.C., de Queiroz, K., Kluge, A.G., and Rowe, T. (1989), "Tetrapod Phylogeny", in B. Fernholm, B., Bremer K., and Jörnvall, H. (PDF), The Hierarchy of Life, Elsevier Science, p. p. 345, http://si-pddr.si.edu/dspace/bitstream/10088/4689/1/VZ_1989GauthieretalHierLife.pdf, retrieved on 2008-09-08
- ↑ Benton, M.J. (March 1983), "Dinosaur Success in the Triassic: a Noncompetitive Ecological Model" (PDF), Quarterly Review of Biology 58 (1), http://palaeo.gly.bris.ac.uk/Benton/reprints/1983success.pdf, retrieved on 2008-09-08
- ↑ Padian, Kevin. (2004). "Basal Avialae". in Weishampel, David B.; Dodson, Peter; & Osmólska, Halszka (eds.). The Dinosauria (Second Edition ed.). Berkeley: University of California Press. pp. 210-231. ISBN 0-520-24209-2.
- ↑ Hou, L., Zhou, Z., Martin, L. D., and Feduccia, A. (October 2002). "A beaked bird from the Jurassic of China". Nature 377: 616–618. doi:. http://www.nature.com/nature/journal/v377/n6550/abs/377616a0.html. Retrieved on 2008-09-08.
- ↑ Clarke, J.A., Zhou, Z., and Zhang, F. (2006). "Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui". Journal of Anatomy 208 (3): 287–308. doi:. http://www3.interscience.wiley.com/journal/118559634/abstract?CRETRY=1&SRETRY=0. Retrieved on 2008-09-08.
- ↑ Ruben, J.A., and Jones, T.D. (2000). "Selective Factors Associated with the Origin of Fur and Feathers". American Zoologist 40 (4): 585–596. doi:. http://icb.oxfordjournals.org/cgi/content/full/40/4/585.
- ↑ Luo, Z-X., Crompton, A.W., and Sun , A-L. (May 2001). "A New Mammaliaform from the Early Jurassic and Evolution of Mammalian Characteristics". Science 292 (5521): 1535–1540. doi:. PMID 11375489. http://www.sciencemag.org/cgi/content/full/292/5521/1535. Retrieved on 2008-09-08.
- ↑ Cifelli, R.L. (November 2001). "Early mammalian radiations". Journal of Paleontology. http://findarticles.com/p/articles/mi_qa3790/is_200111/ai_n8958762/pg_6.
- ↑ Flynn, J.J., Parrish, J.M. Rakotosamimanana, B., Simpson, W.F., and Wyss, A.R. (September 1999). "A Middle Jurassic mammal from Madagascar". Nature 401: 57–60. doi:. http://www.nature.com/nature/journal/v401/n6748/abs/401057a0.html. Retrieved on 2008-09-08.
- ↑ MacLeod N, Rawson PF, Forey PL, Banner FT, Boudagher-Fadel MK, Bown PR, Burnett JA, Chambers, P, Culver S, Evans SE, Jeffery C, Kaminski MA, Lord AR, Milner AC, Milner AR, Morris N, Owen E, Rosen BR, Smith AB, Taylor PD, Urquhart E, Young JR (1997). "The Cretaceous–Tertiary biotic transition". Journal of the Geological Society 154 (2): 265–292. doi:. http://findarticles.com/p/articles/mi_qa3721/is_199703/ai_n8738406/print.
- ↑ Alroy J. (March 1999). "The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation". Systematic biology 48 (1): 107–18. doi:. PMID 12078635.
- ↑ Archibald J.D., and Deutschman D.H. (June 2001). "Quantitative Analysis of the Timing of the Origin and Diversification of Extant Placental Orders". Journal of Mammalian Evolution 8 (2): 107-124. http://www.ingentaconnect.com/content/klu/jomm/2001/00000008/00000002/00342277. Retrieved on 2008-09-24.
- ↑ Simmons, N.B., Seymour,K.L., Habersetzer, J.,and Gunnell, G.F. (February 2008). "Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation". Nature 451: 818–821. doi:.
- ↑ J. G. M. Thewissen, S. I. Madar, and S. T. Hussain (1996). "Ambulocetus natans, an Eocene cetacean (Mammalia) from Pakistan". Courier Forschungsinstitut Senckenberg 191: 1–86.
- ↑ 138.0 138.1 138.2 138.3 Crane, P.R., Friis, E.M., and Pedersen, K.R. (2000), "The Origin and Early Diversification of Angiosperms", in Gee, H., Shaking the Tree: Readings from Nature in the History of Life, University of Chicago Press, pp. 233–250, ISBN 0226284964, http://books.google.co.uk/books?hl=en&lr=&id=ZJe_Dmdbm-QC&oi=fnd&pg=PA233&dq=evolution+flowering+plant+angiosperm&ots=abVpqx_cP8&sig=z1HvmrRLdJP9oPkN0bffbAyriEI#PPA233,M1, retrieved on 2008-09-09
- ↑ 139.0 139.1 139.2 139.3 Crepet, W.L. (November 2000). "Progress in understanding angiosperm history, success, and relationships: Darwin’s abominably "perplexing phenomenon"". Proceedings of the National Academy of Sciences 97 (24): 12939–12941. doi:. PMID 11087846. http://www.pnas.org/content/97/24/12939.full.pdf+html. Retrieved on 2008-09-09.
- ↑ Hughes, W.O.H., Oldroyd, B.P., Beekman, M., and Ratnieks, F.L.W. (2008-05-30). "Ancestral Monogamy Shows Kin Selection Is Key to the Evolution of Eusociality" (html). Science (American Association for the Advancement of Science) 320 (5880): 1213–1216. doi:. PMID 18511689. http://www.sciencemag.org/cgi/content/abstract/320/5880/1213. Retrieved on 2008-08-04.
- ↑ Lovegrove, B.G. (January 1991). "The evolution of eusociality in molerats (Bathyergidae): a question of risks, numbers, and costs". Behavioral Ecology and Sociobiology 28 (1): 37–45. doi:. http://www.springerlink.com/content/k4n52v522l816125/. Retrieved on 2008-09-07.
- ↑ Labandeira, C., and Eble, G.J. (2000), "The Fossil Record of Insect Diversity and Disparity", in Anderson, J., Thackeray, F., van Wyk, B., and de Wit, M. (PDF), Gondwana Alive: Biodiversity and the Evolving Biosphere, Witwatersrand University Press, http://www.santafe.edu/research/publications/workingpapers/00-08-044.pdf, retrieved on 2008-09-07
- ↑ Brunet M., Guy, F., Pilbeam, D., Mackaye, H.T., et al (July 2002). "A new hominid from the Upper Miocene of Chad, Central Africa". Nature 418: 145–151. doi:. http://www.nature.com/nature/journal/v418/n6894/abs/nature00879.html. Retrieved on 2008-09-09.
- ↑ de Heinzelin, J., Clark, J.D., White, T., et al (April 1999). "Environment and Behavior of 2.5-Million-Year-Old Bouri Hominids". Science 284 (5414): 625–629. doi:. PMID 10213682. http://www.sciencemag.org/cgi/content/full/sci;284/5414/625. Retrieved on 2008-09-09.
- ↑ De Miguel, C., and M. Henneberg, M. (2001). "Variation in hominid brain size: How much is due to method?". HOMO - Journal of Comparative Human Biology 52 (1): 3–58. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B7GW4-4DPCHXC-2&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=aef79dbca1f189c885cfe9f36636b131. Retrieved on 2008-09-09.
- ↑ Leakey, Richard (1994). The Origin of Humankind. Science Masters Series. New York, NY: Basic Books. pp. pages 87-89. ISBN 0465053130.
- ↑ Mellars, Paul (2006). "Why did modern human populations disperse from Africa ca. 60,000 years ago?". Proceedings of the National Academy of Sciences 103: 9381. doi:. PMID 16772383. http://www.pnas.org/cgi/reprint/0510792103v1.
- ↑ Benton, M.J. (2004). "6. Reptiles Of The Triassic". Vertebrate Palaeontology. Blackwell. http://www.blackwellpublishing.com/book.asp?ref=0632056371.
- ↑ Van Valkenburgh, B. (1999). "Major patterns in the history of xarnivorous mammals". Annual Review of Earth and Planetary Sciences 26: 463–493. doi:. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.earth.27.1.463.
- ↑ 151.0 151.1 MacLeod, Norman (2001-01-06). "Extinction!". Retrieved on 2008-09-11.
- ↑ Martin, R.E. (1995). "Cyclic and secular variation in microfossil biomineralization: clues to the biogeochemical evolution of Phanerozoic oceans". Global and Planetary Change 11 (1): 1. doi:.
- ↑ Martin, R.E. (1996). "Secular increase in nutrient levels through the Phanerozoic: Implications for productivity, biomass, and diversity of the marine biosphere". Palaios 11: 209–219. doi:.
- ↑ 154.0 154.1 Rohde, R.A., and Muller, R.A. (March 2005). "Cycles in fossil diversity" (PDF). Nature 434: 208-210. http://muller.lbl.gov/papers/Rohde-Muller-Nature.pdf. Retrieved on 2008-09-22.
- ↑ Christopher B. Field, C.B., Behrenfeld, M.J., Randerson, J.T., and Falkowski, P. (July 1998). "Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components". Science 281 (5374): 237-240. doi:. http://www.sciencemag.org/cgi/content/full/sci;281/5374/237. Retrieved on 2008-09-13.
- ↑ Grant, B.S., and Wiseman, L.L. (2002). "Recent History of Melanism in American Peppered Moths". Journal of Heredity 93 (2): 86–90. doi:. ISSN 1465-7333. PMID 12140267. http://jhered.oxfordjournals.org/cgi/content/abstract/93/2/86. Retrieved on 2008-09-11.
- ↑ Levin, B.R., Perrot, V., and Walker, N. (March 2000). "Compensatory Mutations, Antibiotic Resistance and the Population Genetics of Adaptive Evolution in Bacteria". Genetics 154: 985–997. PMID 10757748. http://www.genetics.org/cgi/content/abstract/154/3/985. Retrieved on 2008-09-11.
- ↑ Hawks, J., Wang, E.T., Cochran, G.M., Harpending, H.C. and Moyzis, R.K. (December 2007). "Recent acceleration of human adaptive evolution". Proceedings of the National Academy of Sciences 104 (52): 20753–20758. doi:. PMID 18087044. http://www.pnas.org/content/104/52/20753.full. Retrieved on 2008-09-11.
Further reading
- Cowen, Richard (2004). History of Life (4th edition ed.). Blackwell Publishing Limited. ISBN 978-1405117562.
- Dawkins, Richard (2004). The Ancestor's Tale, A Pilgrimage to the Dawn of Life. Boston: Houghton Mifflin Company. ISBN 0-618-00583-8.
- Dawkins, Richard (1990). The Selfish Gene. Oxford University Press. ISBN 0192860925.
- Smith, John Maynard; Eörs Szathmáry (1997). The Major Transitions in Evolution. Oxfordshire: Oxford University Press. ISBN 0-198-50294-X.
External links
General information
- General information on evolution- Fossil Museum nav.
- Understanding Evolution from University of California, Berkeley
- National Academies Evolution Resources
- Evolution poster- PDF format "tree of life"
- Everything you wanted to know about evolution by New Scientist
- Howstuffworks.com — How Evolution Works
- Synthetic Theory Of Evolution: An Introduction to Modern Evolutionary Concepts and Theories
History of evolutionary thought