Evolution of mammals
- Further information: Evolutionary history of life
The evolution of mammals from synapsids (mammal-like "reptiles") was a gradual process which took approximately 70 million years, beginning in the mid-Permian. By the mid-Triassic, there were many species that looked like mammals, and the first true mammals appeared in the early Jurassic. The earliest known marsupial, Sinodelphys, appeared 125 million years ago in the early Cretaceous, around the same time as Eomaia, the first known eutherian (member of placentals' "parent" group); and the earliest known monotreme, Teinolophos, appeared two million years later. After the Cretaceous-Tertiary extinction wiped out the non-avian dinosaurs (birds are generally regarded as the surviving dinosaurs) and several other mammalian groups, placental and marsupial mammals diversified into many new forms and ecological niches throughout the Tertiary, by the end of which all modern orders had appeared.
From the point of view of phylogenetic nomenclature, mammals are the only surviving synapsids. The synapsid lineage became distinct from the sauropsid ("reptile") lineage in the late Carboniferous period, between 320 and 315 million years ago,[1] and were the most common and largest land vertebrates of the Permian period.[2] But in the Triassic period a previously obscure group of sauropsids, the archosaurs, became the dominant vertebrates and one archosaur group, the dinosaurs, dominated the rest of the Mesozoic era. These changes forced the Mesozoic mammaliforms ("nearly mammals") into nocturnal niches, and may have contributed greatly to the development of mammalian traits such as endothermy, hair and a large brain. Later in the Mesozoic mammals spread into other ecological niches, for example aquatic, gliding and even preying on dinosaurs.
Most of the evidence consists of fossils. For many years fossils of Mesozoic mammals and their immediate ancestors were very rare and fragmentary, but since the mid 1990s there have been many important new finds, especially in China. The relatively new techniques of molecular phylogenetics have also shed light on some aspects of mammalian evolution by estimating the timing of important divergence points for modern species. When used carefully, these techniques often, but not always, agree with the fossil record.
Although mammary glands are the signature feature of modern mammals, little is known about the evolution of lactation, and virtually nothing is known about the evolution of another distinctive feature, the neocortex region of the brain. Most study of the evolution of mammals centers around the development of the middle ear bones from components of the ancestral amniote jaw joint. Other much-studied aspects include the evolution of erect limb posture, a bony secondary palate, fur and hair, and warm-bloodedness.
Definition of "mammal"
Living mammal species can be identified by the presence in females of mammary glands which produce milk.
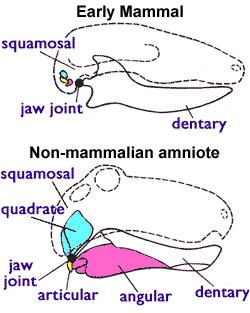
Other features are required when classifying fossils, since mammary glands and other soft-tissue features are not visible in fossils. Paleontologists therefore use a distinguishing feature that is shared by all living mammals (including monotremes) but is not present in any of the early Triassic therapsids ("mammal-like reptiles"): Mammals use two bones for hearing that all other amniotes use for eating. The earliest amniotes had a jaw joint composed of the articular (a small bone at the back of the lower jaw) and the quadrate (a small bone at the back of the upper jaw). All non-mammalian amniotes use this system including lizards, crocodilians, dinosaurs (and their descendants the birds), and therapsids. But mammals have a different jaw joint, composed only of the dentary (the lower jaw bone which carries the teeth) and the squamosal (another small skull bone). And in mammals the quadrate and articular bones have become the incus and malleus bones in the middle ear.[3][4]
Mammals also have a double occipital condyle; they have two knobs at the base of the skull which fit into the topmost neck vertebra, and other vertebrates have a single occipital condyle.[3] But paleontologists use only the jaw joint and middle ear as criteria for identifying fossil mammals, as it would be confusing if they found a fossil that had one feature but not the other (e.g. a mammalian jaw and ear but a non-mammalian single occipital condyle).
Due to the incremental changes in transitional fossils, it has been said
We may again ask the question, What is a mammal? Where we draw the line between reptile and mammal has no biological significance. It is purely a matter of convenience. There are two obvious choices, both immediately following a period of rapid evolution that make as definite a break as we can hope to find.[5]
The ancestry of mammals
Here is a very simplified "family tree" - the text below describes some of the uncertainties and areas of debate.
Tetrapods | +-- Amphibians | `--Amniotes | +-Sauropsids | `-Synapsids | `-Pelycosaurs | `-Therapsids | `-Mammals
Amniotes
The first fully terrestrial vertebrates were amniotes - their eggs had internal membranes which allowed the developing embryo to breathe but kept water in. This allowed amniotes to lay eggs on dry land, while amphibians generally need to lay their eggs in water (a few amphibians, such as the Surinam toad, have evolved other ways of getting round this limitation). The first amniotes apparently arose in the late Carboniferous from the ancestral reptiliomorphs.
Within a few million years two important amniote lineages became distinct: mammals' synapsid ancestors and the sauropsids, from which lizards, snakes, crocodilians, dinosaurs and birds are descended.[1] The earliest known fossils of all these groups date from about 320 to 315M years ago. Unfortunately it is difficult to be sure about when each of them evolved, since vertebrate fossils from the late Carboniferous are very rare, and therefore the actual first occurrences of each of these types of animal might have been considerably earlier.[6]
Synapsids

Synapsid skulls are identified by the distinctive pattern of the holes behind each eye, which served the following purposes:
- made the skull lighter without sacrificing strength.
- saved energy by not using so much bone.
- probably provided attachment points for jaw muscles. Having attachment points further away from the jaw made it possible for the muscles to be longer and therefore to exert a strong pull over a wide range of jaw movement without being stretched or contracted beyond their optimum range.
Early Permian terrestrial fossils indicate that one synapsid group, the pelycosaurs, were the most common land vertebrates of their time and included the largest land animals of the time.[2]
Therapsids
Therapsids descended from pelycosaurs in the middle Permian and took over their position as the dominant land vertebrates. They differ from pelycosaurs in several features of the skull and jaws, including larger temporal fenestrae and incisors which are equal in size.[7]
The therapsids went through a series of stages, beginning with animals which were very like their pelycosaur ancestors and ending with some which could easily be mistaken for mammals:[8]
- gradual development of a bony secondary palate. Most books and articles interpret this as a prequisite for the evolution of mammals' high metabolic rate, because it enabled these animals to eat and breathe at the same time. But some scientists point out that some modern ectotherms use a fleshy secondary palate to separate the mouth from the airway, and that a bony palate provides a surface on which the tongue can manipulate food, facilitating chewing rather than breathing.[9] The interpretation of the bony secondary palate as an aid to chewing also suggests the development of a faster metabolism, since chewing makes it possible to digest food more quickly. In mammals the palate is formed by two specific bones, but various Permian therapsids had other combinations of bones in the right places to function as a palate.
- the dentary gradually becomes the main bone of the lower jaw.
- progress towards an erect limb posture, which would increase the animals' stamina by avoiding Carrier's constraint. But this process was erratic and very slow - for example: all herbivorous therapsids retained sprawling limbs (some late forms may have had semi-erect hind limbs); Permian carnivorous therapsids had sprawling forelimbs, and some late Permian ones also had semi-sprawling hindlimbs. In fact modern monotremes still have semi-sprawling limbs.
- in the Triassic, progress towards the mammalian jaw and middle ear.
- there is plausible evidence of hair in Triassic therapsids, but none for Permian therapsids (see below).
- some scientists have argued that some Triassic therapsids show signs of lactation (see below).
Therapsid family tree
(simplified from [7]; only those which are most relevant to the evolution of mammals are described below)
Therapsids
|
+--Biarmosuchia
|
`--+--Dinocephalia
|
+--Neotherapsida
|
+--Anomodonts
| |
| `--Dicynodonts
|
`--+--Theriodontia
|
+--Gorgonopsia
|
`--+--Therocephalia
|
`--Cynodontia
.
. . . (Mammals, eventually)
Only the dicynodonts, therocephalians and cynodonts survived into the Triassic.
Biarmosuchia
The Biarmosuchia were the most primitive and pelycosaur-like of the therapsids.
Dinocephalians
Dinocephalians ("terrible heads") were large, some as large as a rhinoceros, and included both carnivores and herbivores. Some of the carnivores had semi-erect hindlimbs, but all dinocephalians had sprawling forelimbs. In many ways they were very primitive therapsids, for example they had no secondary palate and their jaws were rather "reptilian".[10]
Theriodonts
The theriodonts ("beast teeth") and their descendants had jaw joints in which the lower jaw's articular bone tightly gripped the skull's very small quadrate bone. This allowed a much wider gape, and one group, the carnivorous gorgonopsians ("gorgon faces"), took advantage of this to develop "sabre teeth". But the theriodont's jaw hinge had a longer term significance - the much reduced size of the quadrate bone was an important step in the development of the mammalian jaw joint and middle ear.
The gorgonopsians still had some primitive features: no bony secondary palate (but other bones in the right places to perform the same functions); sprawling forelimbs; hindlimbs which could operate in both sprawling and erect postures. But the therocephalians ("beast heads"), which appear to have arisen at about the same time as the gorgonopsians, had additional mammal-like features, e.g. their finger and toe bones had the same number of phalanges (segments) as in early mammals (and the same number that primates have, including humans).[11]
Cynodonts
The cynodonts, a theriodont group which also arose in the late Permian, include the ancestors of all mammals - one sub-group, the trithelodonts, is widely regarded as the most likely to contain mammals' ancestor. Cynodonts' mammal-like features include: further reduction in the number of bones in the lower jaw; a secondary bony palate; cheek teeth with a complex pattern in the crowns; the brain filled the endocranial cavity.[12]
Multi-chambered burrows have been found, containing as many as 20 skeletons of the Early Triassic cynodont Trirachodon; the animals are thought to been drowned by a flash flood. The complex, shared burrows indicate that these animals were capable of complex social behaviors.[13]
Triassic takeover
The catastrophic Permian-Triassic mass extinction killed off about 70 percent of terrestrial vertebrate species, and the majority of land plants. As a result:[14]
- Ecosystems and food chains collapsed, and the recovery took about 6 million years.
- The survivors had to re-start the struggle for dominance of their former ecological niches - even the cynodonts, which had seemed on the way to dominance at the end of the Permian.
But the cynodonts lost out to a previously obscure group of sauropsids, the archosaurs (which include the ancestors of crocodilians, dinosaurs and birds). This reversal of fortunes is often called the "Triassic takeover". Several explanations have been offered for it, but the most likely is that the early Triassic was predominantly arid and therefore archosaurs' superior water conservation gave them a decisive advantage (all known sauropsids have glandless skins and excrete uric acid, which requires less water to keep it sufficiently liquid than the urea which mammals excrete and presumably therapsids excreted).[15][8] The Triassic takeover was gradual - in the earliest part of the Triassic cynodonts were the main predators and lystrosaurs were the main herbivores, but by the mid-Triassic archosaurs dominated all the large carnivore and herbivore niches.
But the Triassic takeover may have been a vital factor in the evolution of cynodonts into mammals. The cynodonts' descendants were only able to survive as small, mainly nocturnal insectivores.[12] As a result:
- The therapsid trend towards differentiated teeth with precise occlusion accelerated, because of the need to hold captured arthropods and crush their exoskeletons.
- Nocturnal life required advances in thermal insulation and temperature regulation to enable the ancestors of mammals to be active in the cool of the night.[16]
- Acute senses of hearing and smell became vital.
- This accelerated the development of the mammalian middle ear, and therefore of the mammalian jaw since bones which had been part of the jaw joint became part of the middle ear.
- The increase in the size of the olfactory and auditory lobes of the brain increased brain weight as a total percentage of body weight. Brain tissue requires a disproportionate amount of energy.[17][18] The need for more food to support the enlarged brains increased the pressures for improvements in insulation, temperature regulation and feeding.
- As a side-effect, sight became slightly less important, and this is reflected in the fact that most mammals have poor color vision, including the "lower primates" such as lemurs.[19]
From cynodonts to true mammals
Many uncertainties
While the Triassic takeover probably accelerated the evolution of mammals, it made life more difficult for paleontologists because good fossils of the nearly-mammals are extremely rare, mainly because they were mostly smaller than rats:
- They were largely restricted to environments which are less likely to provide good fossils. The best terrestrial environments for fossilization are floodplains, where seasonal floods quickly cover dead animals in a protective layer of silt which is later compressed into sedimentary rock. But floodplains are dominated by medium to large animals, and the Triassic therapsids and near-mammals could not compete with archosaurs in the medium to large size range.
- Their delicate bones were vulnerable to being destroyed before they could be fossilized - by scavengers (including fungi and bacteria) and by being trodden on.
- Small fossils are harder to spot and more vulnerable to being destroyed by weathering and other natural stresses before they are discovered.
In fact it was said as recently as the 1980s that all the Mesozoic fossils of mammals and near-mammals could be contained in a few shoeboxes - and they were mostly teeth, which are the most durable of all tissues.[20] Since then, the number of Mesozoic fossil mammals has increased, from 116 genera known in 1979 to about 310 in 2007, with an increase in quality such that "at least 18 Mesozoic mammals are represented by nearly complete skeletons".[21]
As a result:
- In many cases it is difficult to assign a Mesozoic mammal or near-mammal fossil to a genus.
- All the available fossils of a genus seldom add up to a complete skeleton, and hence it is difficult to decide which genera are most like each other and therefore most likely to be closely-related. In other words, it becomes very difficult to classify them by means of cladistics, which is the most reliable and least subjective method currently available.
So the evolution of mammals in the Mesozoic is full of uncertainties, although there is no room for doubt that true mammals did first appear in the Mesozoic.
Mammals or mammaliformes?
One result of these uncertainties has been a change in the paleontologists' definition of "mammal". For a long time a fossil was considered a mammal if it met the jaw-ear criterion (the jaw joint consists only of the squamosal and dentary; and the articular and the quadrate bones have become the middle ear's malleus and incus). But more recently paleontologists have usually defined "mammal" as the last common ancestor of monotremes, marsupials and placentals and all of its descendants. So they had to define another clade mammaliformes to accommodate all the animals which are more mammal-like than cynodonts but less closely related to monotremes, marsupials and placentals.[22] Although this now appears to be the majority approach, some paleontologists have resisted it because: it simply moves most of the problems into the new clade without solving them; the clade mammaliformes includes some animals with "mammalian" jaw joints and some with "reptilian" (articular-to-quadrate) jaw joints; and the newer definition of "mammal" and "mammaliformes" depend on last common ancestors of both groups which have not yet been found.[20] Despite these objections, this article follows the majority approach and treats most of the cynodonts' Mesozoic descendants as mammaliformes.
Family tree - cynodonts to mammals
(based on Mammaliformes - Palaeos)
--Cynodonts
|
`--Mammaliformes
|
+--Allotheria
| |
| `--Multituberculates
|
`--+--Morganucodontidae
|
`--+--Docodonta
|
`--+--Hadrocodium
|
`--Symmetrodonta
|
|--Kuehneotheriidae
|
`--Mammals
Multituberculates
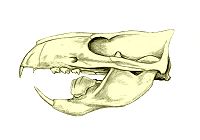
Multituberculates (named for the multiple tubercles on their "molars") are often called the "rodents of the Mesozoic" but this is an example of convergent evolution rather than meaning that they are closely related to the Rodentia. At first sight they look like mammals: their jaw joints consists of only the dentary and squamosal bones, and the quadrate and articular bones are part of the middle ear; their teeth are differentiated, occlude and have mammal-like cusps; they have a zygomatic arch; the structure of the pelvis suggests that they gave birth to tiny helpless young, like modern marsupials. And they lived for over 120 million years (from mid Jurassic, about 160M years ago, to early Oligocene, about 35M years ago), which would have made them the most successful mammals ever. But a closer look shows that they are very different from modern mammals:[22]
- Their "molars" have two parallel rows of tubercles, unlike the tribosphenic (three-peaked) molars of early mammals.
- The chewing action is completely different. Mammals chew with a side-to-side grinding action which means that usually the molars occlude on only one side at a time. Multituberculates' jaws were incapable of side-to-side movement and chewed by dragging the lower teeth backwards against the upper ones as the jaw closed.
- The anterior (forward) part of the zygomatic arch mostly consists of the maxilla (upper jawbone) rather than the jugal, and the jugal is a small bone in a little slot in the maxillary process (extension).
- The squamosal does not form part of the braincase.
- The rostrum (snout) is unlike that of mammals, in fact it looks more like that of a pelycosaur such as Dimetrodon. The multituberculate rostrum is box-like, with the large flat maxillae forming the sides, the nasal the top, and the tall premaxilla at the front.
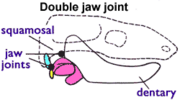
Morganucodontidae
The Morganucodontidae first appeared in the late Triassic, about 205M years ago. They are an excellent example of transitional fossils, since they have both the dentary-squamosal and articular-quadrate jaw joints.[23] They were also one of the first discovered and most thoroughly studied of the mammaliformes, since an unusually large number of morganucodont fossils have been found.
Docodonts

The most notable member of the docodonts is Castorocauda ("beaver tail"), which lived in the mid Jurassic about 164M years ago and was first discovered in 2004 and described in 2006. Castorocauda was not a typical docodont (most were omnivores) and not a true mammal, but it is extremely important in the study of the evolution of mammals because the first find was an almost complete skeleton (a real luxury in paleontology) and it breaks the "small nocturnal insectivore" stereotype:[24]
- It was noticeably larger than most Mesozoic mammal-like fossils - about 17 in (43 cm) from its nose to the tip of its 5-inch (130 mm) tail, and may have weighed 500–800 g (18–28 oz).
- It provides the earliest absolutely certain evidence of hair and fur. Previously the earliest was Eomaia, a true mammal from about 125M years ago.
- It had aquatic adaptations including flattened tail bones and remnants of soft tissue between the toes of the back feet, suggesting that they were webbed. Previously the earliest known semi-aquatic mammal-like animals were from the Eocene, about 110M years later.
- Castorocauda's powerful forelimbs look adapted for digging. This feature and the spurs on its ankles make it resemble the platypus, which also swims and digs.
- Its teeth look adapted for eating fish: the first two molars had cusps in a straight row, which made them more suitable for gripping and slicing than for grinding; and these molars are curved backwards, to help in grasping slippery prey.
Hadrocodium
The consensus family tree above shows Hadrocodium as an "aunt" of true mammals, while symmetrodonts and kuehneotheriids are more closely related to true mammals. But fossils of symmetrodonts and kuehneotheriids are so few and fragmentary that they are poorly understood and may be paraphyletic.[25] On the other hand there are good fossils of Hadrocodium (about 195M years ago in the very early Jurassic) and they have some important features: [26]
- The jaw joint consists only of the squamosal and dentary bones, and the jaw contains no smaller bones to the rear of the dentary, unlike the therapsid design.
- In therapsids and most mammaliformes the eardrum stretched over a trough at the rear of the lower jaw. But Hadrocodium had no such trough, which suggests its ear was part of the cranium, as it is in mammals - and hence that the former articular and quadrate had migrated to the middle ear and become the malleus and incus. On the other hand the dentary has a "bay" at the rear which mammals lack. This suggests that Hadrocodium's dentary bone retained the same shape that it would have had if the articular and quadrate had remained part of the jaw joint, and therefore that Hadroconium or a very close ancestor may have been the first to have a fully mammalian middle ear.
- Therapsids and earlier mammaliforms had their jaw joints very far back in the skull, partly because the ear was at the rear end of the jaw but also had to be close to the brain. This arrangement limited the size of the braincase, because it forced the jaw muscles to run round and over it. Hadrocodium's braincase and jaws were no longer bound to each other by the need to support the ear, and its jaw joint was further forward. In its descendants or those of animals with a similar arrangement, the brain case was free to expand without being constrained by the jaw and the jaw was free to change without being constrained by the need to keep the ear near the brain - in other words it now became possible for mammal-like animals both to develop large brains and to adapt their jaws and teeth in ways that were purely specialized for eating.
The earliest true mammals
This part of the story introduces new complications, since true mammals are the only group which still has living members:
- One has to distinguish between extinct groups and those which have living representatives.
- One often feels compelled to try to explain the evolution of features which do not appear in fossils. This endeavor often involves Molecular phylogenetics, a technique which has become popular since the mid-1980s but is still often controversial because of its assumptions, especially about the reliability of the molecular clock.
Family tree of early true mammals
(based on Mammalia: Overview - Palaeos; X marks extinct groups)
--Mammals | +--Australosphenida | | | +--Ausktribosphenidae X | | | `--Monotremes | `--+--Triconodonta X | `--+--Spalacotheroidea X | `--Cladotheria | |--Dryolestoidea X | `--Theria | +--Metatheria | `--Eutheria
Australosphenida and Ausktribosphenidae
Ausktribosphenidae is a group name that has been given to some rather puzzling finds which:[27]
- appear to have tribosphenic molars, a type of tooth which is otherwise known only in placentals and marsupials.[28]
- come from mid Cretaceous deposits in Australia - but Australia was connected only to Antarctica, and placentals originated in the northern hemisphere and were confined to it until continental drift formed land connections from North America to South America, from Asia to Africa and from Asia to India (the late Cretaceous map at [1] shows how the southern continents are separated).
- are represented only by skull and jaw fragments, which is not very helpful.
Australosphenida is a group which has been defined in order to include the Ausktribosphenidae and monotremes. Asfaltomylos (mid- to late Jurassic, from Patagonia) has been interpreted as a basal australosphenid (animal which: has features shared with both Ausktribosphenidae and monotremes; lacks features which are peculiar to Ausktribosphenidae or monotremes; also lacks features which are absent in Ausktribosphenidae and monotremes) and as showing that australosphenids were wide-spread throughout Gondwanaland (the old Southern hemisphere super-continent).[29]
But recent analysis of Teinolophos suggests Teinolophos (about 115M years ago) was a "crown group" (advanced and relatively specialised) monotreme, so the basal (most primitive) monotremes must have appeared considerably earlier; that some alleged Australosphenids were also "crown group" monotremes (e.g. Steropodon); and that other alleged Australosphenids (e.g. Ausktribosphenos, Bishops, Ambondro, Asfaltomylos) are therefore more closely related to and possibly members of the Therian mammals (group that includes marsupials and placentals, see below).[30]
Monotremes
The earliest known monotreme is Teinolophos, which lived about 123M years ago in Australia. Recent analysis suggest that it was not a basal (primitive, ancestral) monotreme but a full-fledged platypus, and therefore that the platypus and echidna lineages diverged considerably earlier and that basal monotremes were even earlier.[30]
Monotremes have some features which may be inherited from the original amniotes:
- they use the same orifice to urinate, defecate and reproduce ("monotreme" means "one hole") - as lizards and birds also do.
- they lay eggs which are leathery and uncalcified, like those of lizards, turtles and crocodilians.
Unlike in other mammals, female monotremes do not have nipples and feed their young by "sweating" milk from patches on their bellies.
Of course these features are not visible in fossils, and the main characteristics from paleontologists' point of view are:[27]
- a slender dentary bone in which the coronoid process is small or non-existent.
- the external opening of the ear lies at the posterior base of the jaw.
- the jugal bone is small or non-existent.
- a primitive pectoral girdle with strong ventral elements: coracoids, clavicles and interclavicle. Note: therian mammals have no interclavicle.[31]
- sprawling or semi-sprawling forelimbs.
Theria
Theria ("beasts") is a name applied to the hypothetical group from which both metatheria (which include marsupials) and eutheria (which include placentals) descended. Although no convincing fossils of basal therians have been found (just a few teeth and jaw fragments), metatheria and eutheria share some features which one would expect to have been inherited from a common ancestral group:[32]
- no interclavicle.[33]
- coracoid bones non-existent or fused with the shoulder blades to form coracoid processes.
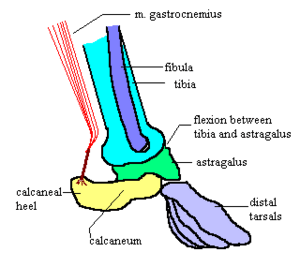
- a type of crurotarsal ankle joint in which: the main joint is between the tibia and astragalus; the calcaneum has no contact with the tibia but forms a heel to which muscles can attach. (The other well-known type of crurotarsal ankle is seen in crocodilians and works differently - most of the bending at the ankle is between the calcaneum and astragalus).
- tribosphenic molars.[28]
Tribosphenic molars have been found in fossils from Madagascar, which indicates that therian mammals are at least .[34]
Metatheria
The living Metatheria are all marsupials ("animals with pouches"). A few fossil genera such as the Mongolian late Cretaceous Asiatherium may be marsupials or members of some other metatherian group(s).[35][36]
The oldest known marsupial is Sinodelphys, found in 125M-year old early Cretaceous shale in China's northeastern Liaoning Province. The fossil is nearly complete and includes tufts of fur and imprints of soft tissues.[37]
Didelphimorphia (common opossums of the Western Hemisphere) first appeared in the late Cretaceous and still have living representatives, probably because they are mostly semi-arboreal unspecialized omnivores.[38]
The best-known feature of marsupials is their method of reproduction:
- The mother develops a kind of yolk sack in her womb which delivers nutrients to the embryo. Embryos of bandicoots, koalas and wombats additionally form placenta-like organs that connect them to the uterine wall, although the placenta-like organs are smaller than in placental mammals and it is not certain that they transfer nutrients from the mother to the embryo.[39]
- Pregnancy is very short, typically 4 to 5 weeks. The embryo is born at a very young age of development, and is usually less than 2 in (5.1 cm) long at birth. It has been suggested that the short pregnancy is necessary to reduce the risk that the mother's immune system will attack the embryo.
- The newborn marsupial uses its forelimbs (with relatively strong hands) to climb to a nipple, which is usually in a pouch on the mother's belly. The mother feeds the baby by contracting muscles over her mammary glands, as the baby is too weak to suck. The newborn marsupial's need to use its forelimbs in climbing to the nipple has prevented the forelimbs from evolving into paddles or wings and has therefore prevented the appearance of aquatic or truly flying marsupials (although there are several marsupial gliders).

Although some marsupials look very like some placentals (the thylacine or "marsupial wolf" is a good example), marsupial skeletons have some features which distinguish them from placentals:[40]
- Some, including the thylacine, have 4 molars. No placentals have more than 3.
- All have a pair of palatal fenestrae, window-like openings on the bottom of the skull (in addition to the smaller nostril openings).
Marsupials also have a pair of marsupial bones (sometimes called "epipubic bones"), which support the pouch in females. But these are not unique to marsupials, since they have been found in fossils of multituberculates, monotremes, and even eutherians - so they are probably a common ancestral feature which disappeared at some point after the ancestry of living placental mammals diverged from that of marsupials.[41][42] Some researchers think the epipubic bones' original function was to assist locomotion by supporting some of the muscles that pull the thigh forwards.[43]
Eutheria
The living Eutheria ("true beasts") are all placentals. But the earliest known eutherian, Eomaia, found in China and dated to 125M years ago, has some features which are more like those of marsupials (the surviving metatherians):[44]
- Epipubic bones extending forwards from the pelvis, which are not found in any modern placental, but are found in marsupials, monotremes and mammaliformes such as multituberculates. In other words, they appear to be an ancestral feature which may even have been present in the earliest placentals.
- A narrow pelvic outlet, which indicates that the young were very small at birth and therefore pregnancy was short, as in modern marsupials. This suggests that the placenta was a later development.
- 5 incisors in each side of the upper jaw. This number is typical of metatherians, and the maximum number in modern placentals is 3, except for homodonts such as the armadillo. But Eomaia's molar to premolar ratio (it has more pre-molars than molars) is typical for eutherians (placentals) and not normal in marsupials.
Eomaia also has a Meckelian groove, a primitive feature of the lower jaw which is not found in modern placental mammals.
These intermediate features are consistent with molecular phylogenetics estimates that the placentals diversified about 110M years ago, 15M years after the date of the Eomaia fossil.
Eomaia also has many features which strongly suggest it was a climber, including: several features of the feet and toes; well-developed attachment points for muscles which are used a lot in climbing; and a tail which is twice as long as the rest of the spine.
Placentals' best-known feature is their method of reproduction:
- The embryo attaches itself to the uterus via a large placenta via which the mother supplies food and oxygen and removes waste products.
- Pregnancy is relatively long and the young are fairly well-developed at birth. In some species (especially herbivores living on plains) the young can walk and even run within an hour of birth.
It has been suggested that the evolution of placental reproduction was made possible by retroviruses which:[45]
- make the interface between the placenta and uterus into a syncytium, i.e. a thin layer of cells with a shared external membrane. This allows the passage of oxygen, nutrients and waste products but prevents the passage of blood and other cells which would cause the mother's immune system to attack the foetus.
- reduce the aggressiveness of the mother's immune system (which is good for the foetus but makes the mother more vulnerable to infections).
From a paleontologist's point of view, eutherians are mainly distinguished by various features of their teeth.[46]
Expansion of ecological niches in the Mesozoic
There is still some truth in the "small, nocturnal insectivores" stereotype but recent finds, mainly in China, show that some mammaliforms and true mammals were larger and had a variety of lifestyles. For example:
- Castorocauda, which lived in the middle Jurassic about 164 million years, was about 42.5 cm (16.7 in) long, weighed 500–800 g (18–28 oz), had limbs which were adapted for swimming and digging and teeth adapted for eating fish.[24]
- Multituberculates, which survived for over 125 million years (from mid Jurassic, about 160M years ago, to early Oligocene, about 35M years ago) are often called the "rodents of the Mesozoic", because they had continuously-growing incisors like those of modern rodents.[22]

- Fruitafossor, from the late Jurassic period about 150 million years ago, was about the size of a chipmunk and its teeth, forelimbs and back suggest that it broke open the nest of social insects to prey on them (probably termites, as ants had not yet appeared).[47]
- Volaticotherium, from the boundary the early Cretaceous about 125M years ago, is the earliest-known gliding mammal and had a gliding membrane which stretched out between its limbs, rather like that of a modern flying squirrel. This also suggests it was active mainly during the day.[48]
- Repenomamus, from the early Cretaceous 130 million years ago, was a stocky, badger-like predator which sometimes preyed on young dinosaurs. Two species have been recognized, one more than 1 m (39 in) long and weighing about 12–14 kg (26–31 lb), the other less than 0.5 m (20 in) long and weighing 4–6 kg (8.8–13 lb).[49][50]
Evolution of major groups of living mammals
There are currently vigorous debates between traditional paleontologists ("fossil-hunters") and molecular phylogeneticists about how and when the true mammals diversified, especially the placentals. Generally the traditional paelontologists date the appearance of a particular group by the earliest known fossil whose features make it likely to be a member of that group, while the molecular phylogeneticists suggest that each lineage diverged earlier (usually in the Cretaceous) and that the earliest members of each group were anatomically very similar to early members of other groups and differed only in their genes. These debates extend to the definition of and relationships between the major groups of placentals - the controversy about Afrotheria is a good example.
Fossil-based family tree of placental mammals
Here is a very simplified version of a typical family tree based on fossils, based on Cladogram of Mammalia - Palaeos. It tries to show the nearest thing there is at present to a consensus view, but some paleontologists have very different views, for example:[51]
- The most common view is that placentals originated in the southern hemisphere, but some paleontologists argue that they first appeared in Laurasia (old supercontinent containing modern Asia, N. America and Europe).
- Paleontologists differ about when the first placentals appeared, with estimates ranging from 20M years before the end of the Cretaceous to just after the end of the Cretaceous. And molecular biologists argue for a much earlier origin.
- Most paleontologists suggest that placentals should be divided into Xenarthra and the rest, but a few think these animals diverged later.
For the sake of brevity and simplicity the diagram omits some extinct groups in order to focus on the ancestry of well-known modern groups of placentals - X marks extinct groups. The diagram also shows the following:
- the age of the oldest known fossils in many groups, since one of the major debates between traditional paleontologists and molecular phylogeneticists is about when various groups first became distinct.
- well-known modern members of most groups.
--Eutheria | +--Xenarthra (Paleocene) | (armadillos, anteaters, sloths) | `--+--Pholidota (early Eocene) | (pangolins) | `--Epitheria (late Cretaceous) | |--(some extinct groups) X | `--+--Insectivora (late Cretaceous) | (hedgehogs, shrews, moles, tenrecs) | `--+--+--Anagalida | | | | | +--Zalambdalestidae X (late Cretaceous) | | | | | `--+--Macroscelidea (late Eocene) | | | (elephant shrews) | | | | | `--+--Anagaloidea X | | | | | `--Glires (early Paleocene) | | | | | +--Lagomorpha (Eocene) | | | (rabbits, hares, pikas) | | | | | `--Rodentia (late Paleocene) | | (mice & rats, squirrels, | | porcupines) | | | `--Archonta | | | |--+--Scandentia (mid [Eocene]) | | | (tree shrews) | | | | | `--Primatomorpha | | | | | +--Plesiadapiformes X | | | | | `--Primates (early Paleocene) | | (tarsiers, lemurs, monkeys, | | apes, humans) | | | `--+--Dermoptera (late Eocene) | | (colugos) | | | `--Chiroptera (late Paleocene) | (bats) | `--+--Ferae (early Paleocene) | (cats, dogs, bears, seals) | `--Ungulatomorpha (late Cretaceous) | +--Eparctocyona (late Cretaceous) | | | +--(some extinct groups) X | | | `--+--Arctostylopida X (late Paleocene) | | | `--+--Mesonychia X (mid Paleocene) | | (predators / scavengers, | | but not closely related | | to modern carnivores) | | | `--Cetartiodactyla | | | +--Cetacea (early Eocene) | | (whales, dolphins, porpoises) | | | `--Artiodactyla (early Eocene) | (even-toed ungulates: | pigs, hippos, camels, | giraffes, cattle, deer) | `--Altungulata | +--Hilalia X | `--+--+--Perissodactyla (late Paleocene) | | (odd-toed ungulates: | | horses, rhinos, tapirs) | | | `--Tubulidentata (early Miocene) | (aardvarks) | `--Paenungulata ("not quite ungulates") | +--Hyracoidea (early Eocene) | (hyraxes) | `--+--Sirenia (early Eocene) | (manatees, dugongs) | `--Proboscidea (early Eocene) (elephants)
This family tree contains some surprises and puzzles. For example:
- The closest living relatives of cetaceans (whales, dolphins, porpoises) are artiodactyls, hoofed animals which are almost all pure vegetarians.
- Bats are fairly close relatives of primates.
- The closest living relatives of elephants are the aquatic sirenians, while their next relatives are hyraxes, which look more like well-fed guinea pigs.
- There is little correspondence between the structure of the family (what was descended from what) and the dates of the earliest fossils of each group. For example the earliest fossils of perissodactyls (the living members of which are horses, rhinos and tapirs) date from the late Paleocene but the earliest fossils of their "sister group" the Tubulidentata date from the early Miocene, nearly 50M years later. Paleontologists are fairly confident about the family relationships, which are based on cladistic analyses, and believe that fossils of the ancestors of modern aardvarks have simply not been found yet.
Family tree of placental mammals according to molecular phylogenetics
Molecular phylogenetics uses features of organisms' genes to work out family trees in much the same way as paleontologists do with features of fossils - if two organisms' genes are more similar to each other than to those of a third organism, the two organisms are more closely related to each other than to the third.
Molecular phylogeneticists have proposed a family tree which is very different from the one with which paleontologists are familiar. Like paleontologists, molecular phylogeneticists have different ideas about various details, but here is a typical family tree according to molecular phylogenetics:[52][53] Note that the diagram shown here omits extinct groups, as one cannot extract DNA from fossils.
--Eutheria
|
+--Atlantogenata ("born round the Atlantic ocean")
| |
| +--Xenarthra (armadillos, anteaters, sloths)
| |
| `--Afrotheria
| |
| +--Afroinsectiphilia
| | (golden moles, tenrecs, otter shrews)
| |
| +--unnamed
| | |
| | +--Macroscelidea (elephant shrews)
| | |
| | `--Tubulidentata (aardvarks)
| |
| `--Paenungulata ("not quite ungulates")
| |
| +--Hyracoidea (hyraxes)
| |
| +--Proboscidea (elephants)
| |
| `--Sirenia (manatees, dugongs)
|
`--Boreoeutheria ("northern true / placental mammals")
|
+--Laurasiatheria
| |
| +--Erinaceomorpha (hedgehogs, gymnures)
| |
| +--Soricomorpha (moles, shrews, solenodons)
| |
| +--Cetartiodactyla
| | (cetaceans and even-toed ungulates)
| |
| `--Pegasoferae
| |
| +--Pholidota (pangolins)
| |
| +--Chiroptera (bats)
| |
| +--Carnivora (cats, dogs, bears, seals)
| |
| `--Perissodactyla (horses, rhinos, tapirs).
|
`--Euarchontoglires
|
+--Glires
| |
| +--Lagomorpha
| | (rabbits, hares, pikas)
| |
| `--Rodentia (late Paleocene)
| (mice & rats, squirrels, porcupines)
|
`--Euarchonta
|
|--Scandentia (tree shrews)
|
|--Dermoptera (colugos)
|
`--Primates
(tarsiers, lemurs, monkeys, apes)
Here are the most significant of the many differences between this family tree and the one familiar to paleontologists:
- The top-level division is between Atlantogenata and Boreoeutheria, instead of between Xenarthra and the rest. But some molecular phylogeneticists have proposed a 3-way top-level split between Xenarthra, Afrotheria and Boreoeutheria.
- Afrotheria contains several groups which are only distantly related according to the paleontologists' version: Afroinsectiphilia ("African insectivores"), Tubulidentata (aardvarks, which paleontologists regard as much closer to odd-toed ungulates than to other members of Afrotheria), Macroscelidea (elephant shrews, usually regarded as close to rabbits and rodents). The only members of Afrotheria which paleontologists would regard as closely related are Hyracoidea (hyraxes), Proboscidea (elephants) and Sirenia (manatees, dugongs).
- Insectivores are split into 3 groups: one is part of Afrotheria and the other two are distinct sub-groups within Boreoeutheria.
- Bats are closer to Carnivora and odd-toed ungulates than to primates and Dermoptera (colugos).
- Perissodactyla (odd-toed ungulates) are closer to Carnivora and bats than to Artiodactyla (even-toed ungulates).
The grouping together of the Afrotheria has some geological justification. All surviving members of the Afrotheria originate from South American or (mainly) African lineages – even the Indian elephant, which diverged from an African lineage about .[54]. As Pangaea broke up Africa and South America separated from the other continents less than 150M years ago, and from each other between 100M and 80M years ago.[55][56] The earliest known eutherian mammal is Eomaia, from about 125M years ago. So it would not be surprising if the earliest eutherian immigrants into Africa and South America were isolated there and radiated into all the available ecological niches.
Nevertheless these proposals have been controversial. Paleontologists naturally insist that fossil evidence must take priority over deductions from samples of the DNA of modern animals. More surprisingly, these new family trees have been criticised by other molecular phylogeneticists, sometimes quite harshly:[57]
- Mitochondrial DNA's mutation rate in mammals varies from region to region - some parts hardly ever change and some change extremely quickly and even show large variations between individuals within the same species.[58][59]
- Mammalian mitochondrial DNA mutates so fast that it causes a problem called "saturation", where random noise drowns out any information that may be present. If a particular piece of mitochondrial DNA mutates randomly every few million years, it will have changed several times in the 60 to 75M years since the major groups of placental mammals diverged.[60]
Timing of placental evolution
Recent molecular phylogenetic studies suggest that most placental orders diverged about 100M to 85M years ago, but that modern families first appeared in the late Eocene and early Miocene.[61]
Some paleontologists object that no placental fossils have been found from before the end of the Cretaceous - for example Maelestes gobiensis, from about 75M years ago, is a eutherian but not a true placental.[62] Many Cretaceous fossil sites contain well-preserved lizards, salamanders, birds, and mammals, but not the modern forms of mammals. It is likely that they simply did not exist, and that the molecular clock runs fast during major evolutionary radiations.[63] On the other hand there is fossil evidence from of hoofed animals which may be ancestors of modern ungulates.[64]
Fossils of the earliest members of most modern groups date from the Paleocene, a few date from later and very few from the Cretaceous, before the extinction of the dinosaurs. But some paleontologists, influenced by molecular phylogenetic studies, have used statistical methods to extrapolate backwards from fossils of members of modern groups and concluded that primates arose in the late Cretaceous.[65] However statistical studies of the fossil record confirm that mammals were restricted in size and diversity right to the end of the Cretaceous, and rapidly grew in size and diversity during the Early Paleocene.[66][67]
Evolution of mammalian features
Jaws and middle ears
See also Evolution of mammalian auditory ossicles
Hadrocodium, whose fossils date from the early Jurassic, provides the first clear evidence of fully mammalian jaw joints and middle ears, in which the jaw joint is formed by the dentary and squamosal bones while the articular and quadrate move to the middle ear, where they are know as the incus and malleus. Curiously it is usually classified as a member of the mammaliformes rather than a as a true mammal.
One analysis of the monotreme Teinolophos suggested that this animal had a pre-mammalian jaw joint formed by the angular and quadrate bones and that the typical mammalian middle ear evolved twice independently, in monotremes and in therian mammals, but this idea has been disputed.[68] In fact 2 of the suggestion's authors co-authored a later paper which reinterpreted the same features as evidence that Teinolophos was a full-fledged platypus, which means it would have had a mammalian jaw joint and middle ear.[30]
Milk production (lactation)
It has been suggested that lactation's original function was to keep eggs moist. Much of the argument is based on monotremes (egg-laying mammals):[69][70][71]
- Monotremes do not have nipples but secrete milk from a hairy patch on their bellies.
- During incubation, monotremes' eggs are covered in a sticky substance whose origin is not known. Before the eggs are laid, their shells have only three layers. Afterwards a fourth layer appears, and its composition is different from that of the original three. The sticky substance and the fourth layer may be produced by the mammary glands.
- If so, that may explain why the patches from which monotremes secrete milk are hairy - it is easier to spread moisture and other substances over the egg from a broad, hairy area than from a small, bare nipple.
Hair and fur
The first clear evidence of hair or fur is in fossils of Castorocauda, from 164M years ago in the mid Jurassic.
From 1955 onwards some scientists have interpreted the foramina (passages) in the maxillae (upper jaws) and premaxillae (small bones in front of the maxillae) of cynodonts as channels which supplied blood vessels and nerves to vibrissae (whiskers), and suggested that this was evidence of hair or fur.[72][73] But foramina do not necessarily show that an animal had vibrissae - for example the modern lizard Tupinambis has foramina which are almost identical to those found in the non-mammalian cynodont Thrinaxodon.[9][74]
Erect limbs
The evolution of erect limbs in mammals is incomplete - living and fossil monotremes have sprawling limbs. In fact some scientists think that the parasagittal (non-sprawling) limb posture is a synapomorphy (distinguishing characteristic) of the Boreosphenida, a group which contains the Theria and therefore includes the last common ancestor of modern marsupials and placentals - and therefore that all earlier mammals had sprawling limbs.[75]
Sinodelphys (the earliest known marsupial) and Eomaia (the earliest known eutherian) lived about 125M years ago, so erect limbs must have evolved before then.
Warm-bloodedness
"Warm-bloodedness" is a complex and rather ambiguous term, because it includes some or all of the following:
- Endothermy, i.e. the ability to generate heat internally rather than via behaviors such as basking or muscular activity.
- Homeothermy, i.e. maintaining a fairly constant body temperature.
- Tachymetabolism, i.e. maintaining a high metabolic rate, particularly when at rest. This requires a fairly high and stable body temperature, since: biochemical processes run about half as fast if an animal's temperature drops by 10°C; most enzymes have an optimum operating temperature and their efficiency drops rapidly outside the preferred range.
Since we can't know much about the internal mechanisms of extinct creatures, most discussion focuses on homeothermy and tachymetabolism.
Modern monotremes have a lower body temperature and more variable metabolic rate than marsupials and placentals.[76] So the main question is when a monotreme-like metabolism evolved in mammals. The evidence found so far suggests Triassic cynodonts may have had fairly high metabolic rates, but is not conclusive.
Respiratory turbinates
Modern mammals have respiratory turbinates, convoluted structures of thin bone in the nasal cavity. These are lined with mucous membranes which warm and moisten inhaled air and extract heat and moisture from exhaled air. An animal with respiratory turbinates can maintain a high rate of breathing without the danger of drying its lungs out, and therefore may have a fast metabolism. Unfortunately these bones are very delicate and therefore have not yet been found in fossils. But rudimentary ridges like those which support respiratory turbinates have been found in Triassic therapsids such as Thrinaxodon and Diademodon, which suggests that they may have had fairly high metabolic rates. [77] [78][72]
Bony secondary palate
Mammals have a secondary bony palate which separates the respiratory passage from the mouth, allowing them to eat and breathe at the same time. Secondary bony palates have been found in the more advanced cynodonts and have been used as evidence of high metabolic rates.[72][73][79] But some cold-blooded vertebrates have secondary bony palates (crocodilians and some lizards), while birds, which are warm-blooded, do not have them.[9]
Diaphragm
A muscular diaphragm helps mammals to breathe, especially during strenuous activity. For a diaphragm to work, the ribs must not restrict the abdomen, so that expansion of the chest can be compensated for by reduction in the volume of the abdomen and vice versa. The advanced cynodonts have very very mammal-like rib cages, with greatly reduced lumbar ribs. This suggests that these animals had diaphragms, were capable of strenuous activity for fairly long periods and therefore had high metabolic rates.[72][73] On the other hand these mammal-like rib cages may have evolved to increase agility.[9] But the movement of even advanced therapsids was "like a wheelbarrow", with the hindlimbs providing all the thrust while the forelimbs only steered the animal, in other words advanced therapsids were not as agile as either modern mammals or the early dinosaurs.[80] So the idea that the main function of these mammal-like rib cages was to increase agility is doubtful.
Limb posture
The therapsids had sprawling forelimbs and semi-erect hindlimbs.[81][73] This suggests that Carrier's constraint would have made it rather difficult for them to move and breathe at the same time, but not as difficult as it is for animals such as lizards which have completely sprawling limbs.[82] But cynodonts (advanced therapsids) had costal plates which stiffened the rib cage and therefore may have reduced sideways flexing of the trunk while moving, which would have made it a little easier for them to breathe while moving .[83] These facts suggest that advanced therapsids were significantly less active than modern mammals of similar size and therefore may have had slower metabolisms.
Insulation (hair and fur)
Insulation is the "cheapest" way to maintain a fairly constant body temperature. So possession of hair of fur would be good evidence of homeothermy but would not be such strong evidence of a high metabolic rate.[84] [85]
The first clear evidence of hair or fur is in fossils of Castorocauda, from 164M years ago in the mid Jurassic;[24] arguments that advanced therapsids had hair are unconvincing.
References
- ↑ 1.0 1.1 "Amniota - Palaeos".
- ↑ 2.0 2.1 "Synapsida overview - Palaeos".
- ↑ 3.0 3.1 Mammalia: Overview - Palaeos
- ↑ Cowen, R. (2000). History of Life. Oxford: Blackwell Science. pp. 432.
- ↑ K. A. Kermack, Frances Mussett and H. W. RIgney (January 1981). "The skull of Morganucodon". Zoological Journal of the Linnean Society 71 (1): page 148. doi:.
- ↑ "Synapsida: Varanopseidae - Palaeos".
- ↑ 7.0 7.1 "Therapsida - Palaeos".
- ↑ 8.0 8.1 Kermack; Kermack (1984). The evolution of mammalian characters. Croom Helm. ISBN 079915349.
- ↑ 9.0 9.1 9.2 9.3 Bennett, A.F.; Ruben, J.A. (1986), "The metabolic and thermoregulatory status of therapsids", in Hotton III, N; MacLean, P.D.; Roth, J.J. et al., The ecology and biology of mammal-like reptiles, Washington: Smithsonian Institution Press, Washington, pp. 207–218
- ↑ "Dinocephalia - Palaeos".
- ↑ "Theriodontia - Paleos".
- ↑ 12.0 12.1 "Cynodontia Overview - Palaeos".
- ↑ Groenewald, G.H., Welman, J. and MacEachern, J.A. (April 2001). "Vertebrate Burrow Complexes from the Early Triassic Cynognathus Zone (Driekoppen Formation, Beaufort Group) of the Karoo Basin, South Africa". Palaios 16 (2): 148–160. doi:. http://palaios.sepmonline.org/cgi/content/abstract/16/2/148. Retrieved on 2008-07-07.
- ↑ "Olenekian Age of the Triassic - Palaeos".
- ↑ "The Triassic Period - Palaeos".
- ↑ Ruben, J.A., and Jones, T.D. (2000). "Selective Factors Associated with the Origin of Fur and Feathers". American Zoologist 40 (4): 585–596. doi:. http://icb.oxfordjournals.org/cgi/content/full/40/4/585.
- ↑ Raichle, M.E.; Gusnard, D.A. (August 6, 2002). "Appraising the brain's energy budget". PNAS 99 (16): 10237–10239. doi:. http://www.pnas.org/cgi/content/full/99/16/10237.
- ↑ "Brain power". New Scientist (2006).
- ↑ Travis, J (October 2003). "Visionary research: scientists delve into the evolution of color vision in primates". Science News 164 (15). http://findarticles.com/p/articles/mi_m1200/is_15_164/ai_110266608.
- ↑ 20.0 20.1 Cifelli, R.L. (November 2001). "Early mammalian radiations". Journal of Paleontology. http://findarticles.com/p/articles/mi_qa3790/is_200111/ai_n8958762/pg_6.
- ↑ Luo, Z.-X. (2007). "Transformation and diversification in early mammal evolution". Nature 450 (7172): 1011–1019. doi:.
- ↑ 22.0 22.1 22.2 "Mammaliformes - Palaeos".
- ↑ "Morganucodontids & Docodonts - Palaeos".
- ↑ 24.0 24.1 24.2 Ji, Q.; Luo, Z-X, Yuan, C-X, and Tabrum, A.R. (February 2006). "A Swimming Mammaliaform from the Middle Jurassic and Ecomorphological Diversification of Early Mammals". Science 311 (5764): 1123. doi:. PMID 16497926. http://www.sciencemag.org/cgi/content/abstract/311/5764/1123. See also the news item at "Jurassic "Beaver" Found; Rewrites History of Mammals".
- ↑ "Symmetrodonta - Palaeos".
- ↑ Luo, Z-X., Crompton, A.W., and Sun , A-L. (May 2001). "A New Mammaliaform from the Early Jurassic and Evolution of Mammalian Characteristics". Science 292 (5521): 1535–1540. doi:. http://www.sciencemag.org/cgi/content/full/292/5521/1535. Retrieved on 2008-09-08.
- ↑ 27.0 27.1 "Mammalia - Palaeos".
- ↑ 28.0 28.1 Jacobs, L.L., Winkler, D.A., and Murry P.A. (01 July 1989). "Modern Mammal Origins: Evolutionary Grades in the Early Cretaceous of North America". Proceedings of the National Academy of Sciences of the USA 86 (13): 4992–4995. doi:. http://links.jstor.org/sici?sici=0027-8424%2819890701%2986%3A13%3C4992%3AMMOEGI%3E2.0.CO%3B2-9&size=LARGE&origin=JSTOR-enlargePage.
- ↑ Rauhut, O.W.M., Martin, T., Ortiz-Jaureguizar, E. and Puerta, P. (14 March 2002). "A Jurassic mammal from South America". Nature 416 (416): 165–168. doi:. http://www.nature.com/nature/journal/v416/n6877/full/416165a.html.
- ↑ 30.0 30.1 30.2 Rowe, T., Rich, T.H., Vickers-Rich, P., Springer, M., and Woodburne, M.O. (January 2008). "The oldest platypus and its bearing on divergence timing of the platypus and echidna clades". Proceedings of the National Academy of Sciences 105 (4): 1238–1242. doi:. http://www.pnas.org/cgi/content/full/105/4/1238.
- ↑ "Appendicular Skeleton".
- ↑ "Mammalia: Spalacotheroidea & Cladotheria - Palaeos".
- ↑ "Appendicular Skeleton".
- ↑ Flynn, J.J., Parrish, J.M. Rakotosamimanana, B., Simpson, W.F., and Wyss, A.R. (September 1999). "A Middle Jurassic mammal from Madagascar". Nature 401: 57–60. doi:. http://www.nature.com/nature/journal/v401/n6748/abs/401057a0.html. Retrieved on 2008-09-08.
- ↑ "Metatheria - Palaeos".
- ↑ Szalay, F.S.; Trofimov, B.A. (1996), "The Mongolian Late Cretaceous Asiatherium, and the early phylogeny and paleobiogeography of Metatheria" (– Scholar search), Journal of Vertebrate Paleontology 16 (3): 474–509, http://www.vertpaleo.org/jvp/16-474-509.html
- ↑ "Oldest Marsupial Fossil Found in China". National Geographic News (2003-12-15).
- ↑ "Didelphimorphia - Palaeos".
- ↑ "Family Peramelidae (bandicoots and echymiperas)".
- ↑ "Species is as species does... Part II".
- ↑ "Marsupials".
- ↑ Novacek, M.J.; Rougier, G.W.; Wible, J.R.; McKenna, M.C.; Dashzeveg, D; Horovitz, I (1997), "Epipubic bones in eutherian mammals from the late Cretaceous of Mongolia", Nature 389 (6650): 440–441, doi:, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9333234&dopt=Abstract
- ↑ White, T.D. (August 9 1989). "An analysis of epipubic bone function in mammals using scaling theory". Jornal of Theoretical Biology 139 (3): 343–57. doi:. http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&uid=2615378&cmd=showdetailview&indexed=google.
- ↑ "Eomaia scansoria: discovery of oldest known placental mammal".
- ↑ Fox, D (1999), "Why we don't lay eggs", New Scientist, http://www.newscientist.com/article/mg16221904.800-why-we-dont-lay-eggs.html
- ↑ "Eutheria - Palaeos".
- ↑ Luo, Z.-X., Wible, J.R. (2005). "A Late Jurassic Digging Mammal and Early Mammal Diversification". Science 308: 103–107.. doi:.
- ↑ Meng, J., Hu, Y., Wang, Y., Wang, X., Li, C. (December 2006). "A Mesozoic gliding mammal from northeastern China". Nature 444 (7121): 889–893. doi:. http://www.nature.com/nature/journal/v444/n7121/abs/nature05234.html.
- ↑ Li, J., Wang, Y., Wang, Y., Li, C. (2000). "A new family of primitive mammal from the Mesozoic of western Liaoning, China". Chinese Science Bulletin 46 (9): 782–785. abstract, in English
- ↑ Hu, Y., Meng, J., Wang, Y., Li, C. (2005). "Large Mesozoic mammals fed on young dinosaurs". Nature 433: 149–152. doi:. http://www.nature.com/cgi-taf/DynaPage.taf?file=/nature/journal/v433/n7022/abs/nature03102_fs.html&dynoptions=doi1105989664.
- ↑ Wible, J.R., Rougier, G.W., Novacek, M.J., and Asher, R.J. (2007). "Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary". Nature 447 (447): 1003–1006. doi:. http://www.nature.com/nature/journal/v447/n7147/full/nature05854.html.
- ↑ Murphy, W.J., Eizirik, E., Springer, M.S et al (14 December 2001). "Resolution of the Early Placental Mammal Radiation Using Bayesian Phylogenetics". Science 294 (5550): 2348–2351. doi:.
- ↑ Kriegs, J.O., Churakov, G., Kiefmann, M., et al (2006). "Retroposed Elements as Archives for the Evolutionary History of Placental Mammals". PLoS Biol 4 (4): e91. doi:. (pdf version)
- ↑ "Scientists map elephant evolution". Retrieved on 2008-08-11.
- ↑ Historical perspective (the Dynamic Earth, USGS)
- ↑ Cretaceous map
- ↑ Insectivora Overview - Palaeos
- ↑ Springer, M.S.; Douzery, E. (1996). "Secondary Structure and patterns of evolution among mammalian mitochondrial 12S rRNA molecules". J. Mol. Evol. 43: 357–373. doi:.
- ↑ Springer, M.S.; Hollar, L.J.; Burk, A. (1995). "Compensatory substitutions and the evolution of the mitochondrial 12S rRNA gene in mammals". Mol. Biol. Evol. 12: 1138–1150.
- ↑ Li, W-H (1997). Molecular Evolution. Sinauer Associates.
- ↑ Bininda-Emonds, O.R.P.; Cardillo, M.; Jones, K.E.; 'et al' (2007). "The delayed rise of present-day mammals". Nature (446): 507–511. http://scienceblogs.com/pharyngula/2007/03/dont_blame_the_dinosaurs.php.
- ↑ Dinosaur Extinction Spurred Rise of Modern Mammals
- ↑ Benton, M.J. (December 1999). "Early origins of modern birds and mammals: molecules vs. morphology". BioEssays 21: 1043–1051. doi:.
- ↑ Archibald, J.D. (May 1996). "Fossil Evidence for a Late Cretaceous Origin of "Hoofed" Mammals". Science 272 (5265): 1150–1153. doi:. http://www.sciencemag.org/cgi/content/abstract/272/5265/1150. Retrieved on 2008-09-08.
- ↑ Martin, R.D.; Soligo, C.; Tavaré, S. (2007). "Primate Origins: Implications of a Cretaceous Ancestry" (PDF). Folia Primatologica 78 (78): 277–296. doi:. http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowPDF&ArtikelNr=105145&Ausgabe=233328&ProduktNr=223842&filename=105145.pdf. - a similar paper by these authors is free online at New light on the dates of primate origins and divergence
- ↑ Alroy J. (March 1999). "The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation". Systematic biology 48 (1): 107–18. doi:. PMID 12078635.
- ↑ Archibald J.D., and Deutschman D.H. (June 2001). "Quantitative Analysis of the Timing of the Origin and Diversification of Extant Placental Orders". Journal of Mammalian Evolution 8 (2): 107-124. http://www.ingentaconnect.com/content/klu/jomm/2001/00000008/00000002/00342277. Retrieved on 2008-09-24.
- ↑ Rich, T.H., Hopson, J.A., Musser, A.M., Flannery., T.GF., and Vickers-Rich, P. (11 February 2005). "Independent Origins of Middle Ear Bones in Monotremes and Therians". Science 307 (5711): 910–914. doi:. http://www.sciencemag.org/cgi/content/abstract/307/5711/910. For other opinions see "Technical comments" linked from same Web page
- ↑ Oftedal, O.T. (2002). "The mammary gland and its origin during synapsid evolution". Journal of Mammary Gland Biology and Neoplasia 7 (3): 225–252. doi:.
- ↑ Oftedal, O.T. (2002). The origin of lactation as a water source for parchment-shelled eggs=Journal of Mammary Gland Biology and Neoplasia. 7. pp. 253–266.
- ↑ Lactating on Eggs
- ↑ 72.0 72.1 72.2 72.3 Brink, A.S. (1955). "A study on the skeleton of Diademodon". Palaeontologia Africana 3: 3–39.
- ↑ 73.0 73.1 73.2 73.3 Kemp, T.S. (1982). Mammal-like reptiles and the origin of mammals. London: Academic Press. pp. 363.
- ↑ Estes, R. (1961). "Cranial anatomy of the cynodont reptile Thrinaxodon liorhinus". Bulletin of the Museum of Comparative Zoology: 165–180.
- ↑ Kielan−Jaworowska, Z.; Hurum, J.H.. (2006). "Limb posture in early mammals: Sprawling or parasagittal" (PDF). Acta Palaeontologica Polonica 51 (3): 10237–10239. http://www.app.pan.pl/archive/published/app51/app51-393.pdf. Retrieved on 2008-09-24.
- ↑ Paul, G.S. (1988). Predatory Dinosaurs of the World. New York: Simon and Schuster. pp. 464.
- ↑ Hillenius, W.H. (1992). "The evolution of nasal turbinates and mammalian endothermy". Paleobiology 18 (1): 17–29.
- ↑ Ruben, J. (1995). "The evolution of endothermy in mammals and birds: from physiology to fossils". Annual Review of Physiology 57: 69–95. doi:.
- ↑ McNab, B.K. (1978). "The evolution of endothermy in the phylogeny of mammals". American Naturalist 112: 1–21.
- ↑ Ccowen, R. (2000). History of Life. Oxford: Blackwell Science. pp. 432.
- ↑ Jenkins, F.A., Jr (1971). "The postcranial skeleton of African cynodonts". Bulletin of the Peabody Museum of Natural History (36): 1–216.
- ↑ Pough, F.H; Heiser, J.B.; McFarland, W.N. (1996). Vertebrate Life. New Jersey: Prentice-Hall. pp. 798.
- ↑ Sidor, C.A.; Hopson, J.A. (1998). "Ghost lineages and "mammalness": assessing the temporal pattern of character acquisition in the Synapsida". Paleobiology (24): 254–273.
- ↑ Schmidt-Nielsen, K. (1975). Animal physiology: Adaptation and environment. Cambridge: Cambridge University Press. pp. 699.
- ↑ Withers, P.C. (1992). Comparative Animal Physiology. Fort Worth: Saunders College. pp. 949.
Bibliography
- Robert L. Carroll, Vertebrate Paleontology and Evolution, W. H. Freeman and Company, New York, 1988 ISBN 0-716-71822-7. Chapters XVII through XXI
- Nicholas Hotton III, Paul D. MacLean, Jan J. Roth, and E. Carol Roth, editors, The Ecology and Biology of Mammal-like Reptiles, Smithsonian Institution Press, Washington and London, 1986 ISBN 0-87474-524-1
- T. S. Kemp, The Origin and Evolution of Mammals, Oxford University Press, New York, 2005 ISBN 0-19-850760-7
- Zofia Kielan-Jaworowska, Richard L. Cifelli, and Zhe-Xi Luo, Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure, Columbia University Press, New York, 2004 ISBN 0-231-11918-6. Comprehensive coverage from the first mammals up to the time of the K-T mass extinction.
- Zhe-Xi Luo, "Transformation and diversification in early mammal evolution", Nature volume 450 number 7172 (13 December 2007) pages 1011–1019. doi:10.1038/nature06277. A survey article with 98 references to the scientific literature.
External links
- The Cynodontia covers several aspects of the evolution of cynodonts into mammals, with plenty of references.