Esomeprazole
 |
|
|
Esomeprazole
|
|
| Systematic (IUPAC) name | |
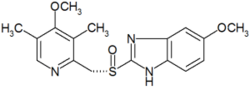
| (S)-5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl) methylsulfinyl]-3H-benzoimidazole |
|
| Identifiers | |
| CAS number | |
| ATC code | A02 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C17H19N3O3S |
| Mol. mass | 345.417 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 50 to 90% |
| Metabolism | Hepatic (CYP2C19, CYP3A4) |
| Half life | 1–1.5 hours |
| Excretion | 80% Renal 20% faecal |
| Therapeutic considerations | |
| Pregnancy cat. |
B3(AU) |
| Legal status | |
| Routes | Oral, IV |
Esomeprazole (pronounced /ɛsoʊˈmɛprəzoʊl/) is a proton pump inhibitor (brand names Sompraz, Zoleri, Nexium, Lucen, Esopral; Axagon in Italy) developed and marketed by AstraZeneca which is used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD) and Zollinger-Ellison syndrome. Esomeprazole is the S-enantiomer of omeprazole (marketed as Losec/Prilosec), and AstraZeneca claims improved efficacy of this single enantiomer product over the racemic mixture of omeprazole. However, this greater efficacy has been disputed, with some claiming it offers no benefit from its older form. (see below). Esomeprazole was the third biggest selling pharmaceutical drug in the world for 2005, totaling US$ 5.7 billion in sales.
Contents |
Pharmacology
Esomeprazole is a proton pump inhibitor which reduces gastric acid secretion through inhibition of H+/K+-ATPase in gastric parietal cells. By inhibiting the functioning of this enzyme, the drug prevents formation of gastric acid.
Clinical use
Use in Helicobacter pylori eradication
Esomeprazole is combined with the antibiotics clarithromycin and amoxicillin (or metronidazole) in the 7-day eradication triple therapy for Helicobacter pylori. Infection by H. pylori is the ive factor in the majority of peptic and duodenal ulcers.
Evidence of efficacy
AstraZeneca claims that esomeprazole provides improved efficacy, in terms of stomach acid control, over racemic omeprazole. Many health professionals have expressed the view that this improvement in efficacy is due to the dose of esomeprazole recommended for therapy rather than any superiority of esomeprazole per se.
An alternative rationale suggested for the use of esomeprazole was the reduction in interindividual variability in efficacy. However the clinical advantage of this hypothesis has not thoroughly been tested in large-scale trials.[Somogyi 2004]
Given the large difference in cost between all other proton pump inhibitors and that of Prilosec-OTC (equivalent to omeprazole 20mg), many physicians recommend a trial of over-the-counter products before beginning more extensive therapies and testing.
Although the (S)-isomer is more potent in humans, the (R)-isomer is more potent in the testing of rats, while the enantiomers are equipotent in dogs. ( "The Organic Chemistry of Drug Design and Drug Action, Richard B. Silverman, page 148)
Dosage forms
Esomeprazole is available as delayed-release capsules in the United States or as delayed release tablets in Australia and Canada (containing esomeprazole magnesium) in strengths of 20 mg and 40 mg; and as esomeprazole sodium for intravenous injection/infusion. Oral esomeprazole preparations are enteric-coated, due to the rapid degradation of the drug in the acidic conditions of the stomach. This is achieved by formulating capsules using the multiple-unit pellet system.
Multiple unit pellet system
Esomeprazole capsules are formulated as a "multiple unit pellet system" (MUPS). Essentially, the capsule consists of extremely small enteric-coated granules (pellets) of the esomeprazole formulation inside an outer shell. When the capsule is immersed in an aqueous solution, as happens when the capsule reaches the stomach, water enters the capsule by osmosis. The contents swell from water absorption causing the shell to burst, releasing the enteric-coated granules. For most patients, the multiple-unit pellet system is of no advantage over conventional enteric-coated preparations. Patients for which the formulation is of benefit include those requiring nasogastric tube feeding and those with difficulty swallowing (dysphagia).
The granules are manufactured in a fluid bed system with small sugar spheres as the starting material. The sugar spheres are sequentially spray-coated with a suspension containing esomeprazole, a protective layer to prevent degradation of the drug in manufacturing, an enteric coating and an outer layer to reduce granule aggregation. The granules are mixed with other inactive excipients and compressed into tablets. Finally, the tablets are film-coated to improve the stability and appearance of the preparation.
Side Effects
Side effects include headache, diarrhea, nausea, gas, stomach pain, constipation, and dry mouth.
Financial impact
Between the launch of esomeprazole in 2001 and 2005, the drug has netted AstraZeneca about $14.4 billion.[1]
Controversy
There has been some controversy about AstraZeneca's behaviour in creating, patenting and marketing of the drug. Critics allege that the drug's successful predecessor Omeprazole is a mixture of two mirror-imaged molecules (esomeprazole and romeprazole), and that the company was trying to "evergreen" its patent by patenting the pure esomeprazole and aggressively marketing to doctors that it is more effective than the mixture, claiming that romeprazole has no beneficial effects on the patient. However, in the acidic environment of the parietal cells both esomeprazole and romeprazole are converted to the same active drug which stops the gastric acid production.
Dr. Marcia Angell, former Editor in Chief of the New England Journal of Medicine, spoke at Harvard Medical School to a German magazine on August 16, 2007 and accused AstraZeneca's scientists of deceptively doctoring their comparative studies such that the difference to Omeprazole would look larger, providing a marketing advantage . For more information, see AstraZeneca's article.
References
- Lind 2004 Lind, T.; Rydberg L., Kyleback A., Jonsson A., Andersson T., Hasselgren T., Holmberg J., Rohss K. (July 2000). "Esomeprazole provides improved acid control vs. omeprazole In patients with symptoms of gastro-oesophageal reflux disease". Alimentary pharmacology & therapeutics 14 (7): 861–867. PMID 10886041.
- Somogyi 2004 Somogyi A., Bochner F., Foster D. (2004). Inside the isomers: the tale of chiral switches. Aust Prescr 27, 47-49.
- Gladwell, Malcolm (2004-10-25). "High Prices: How to think about prescription drugs.", A Critic At Large, The New Yorker. Retrieved on 2006-12-02. This article describes AstraZeneca's strategy for developing Nexium as a follow-on for Losec as the latter approached patent expiry.
Notes
- ^ Gladwell, Malcolm (October 25, 2004). "High Prices: How to think about prescription drugs". The New Yorker. Verified availability August 19, 2007.
- ^ Grill, Markus and Hansen, Hans: "Vorsicht, Pharma! Wie die Industrie Ärzte manipuliert und Patienten täuscht." ('Caution, Pharma! How the industry manipulates physicians and deceives patients.') Published in the 16.08.2007 issue of the magazine "Der Stern" (Germany; pp. 100-107). Available as an e-paper here. }}
- ↑ Financial impact information: 2005, $4.6 billion; 2004, $3.9 billion; 2003, $3.3 billion; 2002, $2 billion; 2001, launch and $580 million.
External links
|
||||||||||||||