Endocarditis
| Endocarditis Classification and external resources |
|
 |
|
|---|---|
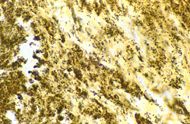
| Bartonella henselae bacilli in cardiac valve of a patient with blood culture-negative endocarditis. The bacilli appear as black granulations. | |
| ICD-10 | I33. |
| ICD-9 | 421 |
| DiseasesDB | 4224 |
| MedlinePlus | 001098 |
| eMedicine | emerg/164 med/671 ped/2511 |
| MeSH | D004696 |
Endocarditis is an inflammation of the inner layer of the heart, the endocardium. It usually involves the heart valves (native or prosthetic valves). Other structures which may be involved include the interventricular septum, the chordae tendinae, the mural endocardium, or even on intracardiac devices. Endocarditis is characterized by a prototypic lesion, the vegetation, which is a mass of platelets, fibrin, microcolonies of microorganisms, and scant inflammatory cells.[1] In the subacute form of infective endocarditis, the vegegation may also include a center of granulomatous tissue, which may fibrose or calcify.[2]
There are multiple ways to classify endocarditis. The simplest classification is based on etiology: either infective or non-infective, depending on whether a microorganism is the source of the inflammation. Regardless, diagnosis of endocarditis is based on the clinical features, investigations such as echocardiogram, as well as any blood cultures demonstrating the presence of endocarditis-causing microorganisms.
Contents |
Infective endocarditis

The valves of the heart do not receive any dedicated blood supply, defensive immune mechanisms (such as white blood cells) cannot direct reach the valves via the bloodstream. If an organism (such as bacteria) attaches to a valve surface and forms a vegetation, the host immune response is blunted. The lack of blood supply to the valves also has implications on treatment, since drugs also have difficulty reaching the infected valve.
Normally, blood flows smoothly through these valves. If they have been damaged - from rheumatic fever, for example - the risk of bacteria attachment is increased.[2]
Classification
Traditionally, infective endocarditis has been clinically divided into acute and subacute[2] (because the patients tend to live longer in subacute as opposed to acute) endocarditis. This classifies both the rate of progression and severity of disease. Thus subacute bacterial endocarditis (SBE) is often due to streptococci of low virulence and mild to moderate illness which progresses slowly over weeks and months and has low propensity to hematogenously seed extracardiac sites, while acute bacterial endocarditis (ABE) is a fulminant illness over days to weeks, and is more likely due to Staphylococcus aureus which has much greater virulence, or disease-producing capacity and frequently causes metastatic infection[2].
This terminology is now discouraged. The terms short incubation (meaning less than about six weeks), and long incubation (greater than about six weeks) are preferred.[3]
Infective endocarditis may also be classified as culture-positive or culture-negative. Culture-negative endocarditis can be due to micro-organisms that require a longer period of time to be identified in the laboratory, such organisms are said to be fastidious because they have demanding growth requirements, or due to absence of an organism as in marantic endocarditis. Some pathogens responsible for culture-negative endocarditis include Aspergillus species, Brucella species, Coxiella burnetii, Chlamydia species, and HACEK bacteria.
Patients who inject narcotics intravenously may introduce infection which will travel to the right side of the heart classically affecting the tricuspid valve, and most often caused by S. aureus.[2] In other patients without a history of intravenous exposure, endocarditis is more frequently left-sided[2].
Another form of endocarditis is nosocomial endocarditis which is when the patient is diagnosed with endocarditis and has had hospital care one month prior to the incident and is usually secondary to IV catheters, Total parenteral nutrition lines, pacemakers, etc.[1]
Finally, the distinction between native-valve endocarditis and prosthetic-valve endocarditis is clinically important. Prosthetic valve endocarditis can be early (< 2 months of valvular surgery) or late (> 2 months of valvular surgery). Early prosthetic valve endocarditis is usually due to intraoperative contamination or a postoperative bacterial contamination which is usually nosocomial in nature. Late prosthetic valve endocarditis is usually due to community acquired microorganisms.[1]
Etiology and pathogenesis
As previously mentioned, altered blood flow around the valves is a risk factor in obtaining endocarditis. The valves may be damaged congenitally, from surgery, by auto-immune mechanisms, or simply as a consequence of old age. The damaged part of a heart valve becomes covered with a blood clot, a condition known as non-bacterial thrombotic endocarditis (NBTE). Altered blood flow, and thus infective endocarditis, are more likely in high pressure areas. Consequently, ventricular septal defects create more susceptibility than atrial septal defects. Damaged vascular endothelium will also promote platelet and fibrin deposition, upon which bacteria can take hold. Valvular lesions are a major cause of such damage, as are jet lesions resulting from ventricular septal defects or patent ductus arteriosus.
In a healthy individual, a bacteremia (where bacteria get into the blood stream through a minor cut or wound) would normally be cleared quickly with no adverse consequences. If a heart valve is damaged and covered with a piece of a blood clot, the valve provides a place for the bacteria to attach themselves and an infection can be established.
In the past bacteremia caused by dental procedures (in most cases due to viridans streptococci, which reside in oral cavity), such as a cleaning or extraction of a tooth was thought to be clinically significant. It is important that a dentist or a dental hygienist is told of any heart problems before commencing treatment. Antibiotics are administered to patients with certain heart conditions as a precaution, although this practice has changed in the US with new American Heart Association guidelines released in 2007[4] and in the UK as of March 2008 due to new NICE guidelines. Everyday tooth brushing and flossing will similarly cause bacteremia, and there is little evidence to support antibiotic prophylaxis for dental treatment which is reflected the current guidelines.
Another group of causes result from a high number of bacteria getting into the bloodstream. Colorectal cancer (mostly Streptococcus bovis), serious urinary tract infections (mostly enterococci), and IV drug (S. aureus) use can all introduce large numbers of bacteria. With a large number of bacteria, even a normal heart valve may be infected. A more virulent organism (such as S. aureus, but see below for others) is usually responsible for infecting a normal valve.
Intravenous drug users tend to get their right-sided heart valves infected because the veins that are injected enter the right side of the heart. The injured valve is most commonly affected when there is a pre-existing disease. (In rheumatic heart disease this is the aortic and the mitral valves, on the left side of the heart).
Other factors that increase the risk of developing infective endocarditis are low levels of white blood cells, immunodeficiency or immunosuppression, malignancy, diabetes, and alcohol abuse[2].
Clinical and pathological features
- Fever, i.e. fever of unknown origin (often spiking caused by septic emboli)
- Continuous presence of micro-organisms in the bloodstream determined by serial collection of blood cultures[2]
- Vegetations on valves on echocardiography, which sometimes can cause a new or changing heart murmur[2], particularly murmurs suggestive of valvular regurgitation
- Vascular phenomena: septic embolism (causing thromboembolic problems such as stroke in the parietal lobe of the brain or gangrene of fingers), Janeway lesions (painless hemorrhagic cutaneous lesions on the palms and soles), intracranial hemorrhage, conjunctival hemorrhage, splinter hemorrhages
- Immunologic phenomena: Glomerulonephritis which allows for blood and albumin to enter the urine[2], Osler's nodes (painful subcutaneous lesions in the distal fingers), Roth's spots on the retina, positive serum rheumatoid factor
Diagnosis
The most important investigation is blood culture. In general, a patient should fulfill the Duke Criteria[5] in order to establish the diagnosis of endocarditis.
As the Duke Criteria rely heavily on the results of echocardiography, research has addressed when to order an echocardiogram by using signs and symptoms to predict occult endocarditis among patients with intravenous drug abuse[6][7][8] and among non drug-abusing patients [9][10]. Unfortunately, this research is over 20 years old and it is possible that changes in the epidemiology of endocarditis and bacteria such as staphylococci make the following estimates incorrectly low.
Among patients who do not use illicit drugs and have a fever in the emergency room, there is a less than 5% chance of occult endocarditis. Mellors in 1987 found no cases of endocarditis nor of staphylococcal bacteremia among 135 febrile patients in the emergency room.[10] The upper confidence interval for 0% of 135 is 5%, so for statistical reasons alone, there is up to a 5% chance of endocarditis among these patients. In contrast, Leibovici found that among 113 non-selected adults admitted to the hospital because of fever there were two cases (1.8% with 95%CI: 0% to 7%) of endocarditis.[9]
Among patients who do use illicit drugs and have a fever in the emergency room, there is about a 10% to 15% prevalence of endocarditis. This estimate is not substantially changed by whether the doctor believes the patient has a trivial explanation for their fever.[8] Weisse found that 13% of 121 patients had endocarditis.[6] Marantz also found a prevalence of endocarditis of 13% among such patients in the emergency room with fever.[8] Samet found a 6% incidence among 283 such patients, but after excluding patients with initially apparent major illness to explain the fever (including 11 cases of manifest endocarditis), there was a 7% prevalence of endocarditis.[7]
Among patients with staphylococcal bacteremia (SAB), one study found a 29% prevalence of endocarditis in community-acquired SAB versus 5% in nosocomial SAB[11]. However, only 2% of strains were resistant to methicillin and so these numbers may be low in areas of higher resistance.
Echocardiography
The transthoracic echocardiogram has a sensitivity and specificity of approximately 65% and 95% if the echocardiographer believes there is 'probable' or 'almost certain' evidence of endocarditis[12][13].
Duke Criteria
Major Criteria
Positive blood culture for infective endocarditis
- Typical microorganism consistent with IE from 2 separate blood cultures, as noted below:
-
- viridans-group streptococci, S. bovis, or HACEK group, or
- community-acquired S. aureus or enterococci, in the absence of a primary focus
- or
- Microorganisms consistent with IE from persistently positive blood cultures defined as:
-
- 2 positive cultures of blood samples drawn >12 hours apart, or
- all of 3 or a majority of 4 separate cultures of blood (with first and last sample drawn 1 hour apart)
Evidence of endocardial involvement Positive echocardiogram for IE defined as :
- oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explanation, or
- abscess, or
- new partial dehiscence of prosthetic valve or new valvular regurgitation (worsening or changing of preexisting murmur not sufficient)
Minor Criteria
- Predisposing factor: known cardiac lesion, recreational drug injection
- Fever >38° C
- Evidence of embolism: pulmonary infarcts, Janeway lesions, conjunctival hemorrhages
- Immunological problems: glomerulonephritis, Osler's nodes
- Positive blood culture (that doesn't meet a major criterion)
- Positive echocardiogram (that doesn't meet a major criterion)
Micro-organisms responsible
Many microorganisms can cause infective endocarditis. These are generally isolated by blood culture, where the patient's blood is removed, and any growth is noted and identified. Alpha-hemolytic streptococci, that are present in the mouth will often be the organism isolated if a dental procedure caused the bacteraemia. If the bacteraemia was introduced through the skin, such as contamination in surgery, during catheterisation, or in an IV drug user, S. aureus is common. A third important cause of endocarditis are bacteria of the genus Enterococcus. These bacteria enter the bloodstream as a consequence of abnormalities in the gastrointestinal or urinary tracts. Enterococci are increasingly recognized as causes of nosocomial or hospital-acquired endocarditis. This contrasts with alpha-haemolytic streptococci and S. aureus which are causes of community-acquired endocarditis.
Some organisms, when isolated, give valuable clues to the cause, as they tend to be specific.
- Candida albicans, a yeast, is associated with IV drug users and the immunocompromised.
- Pseudomonas species, which are very resilient organisms that thrive in water, may contaminate street drugs that have been contaminated with drinking water. P. aeruginosa can infect a child through foot punctures, and can cause both endocarditis and septic arthritis.[14]
- S. bovis and Clostridium septicum, which are part of the natural flora of the bowel, are associated with colonic malignancies. When they present as the causative agent in endocarditis, it usually call for a concomitant colonoscopy due to worries regarding hematogenous spread of bacteria from the colon due to the neoplasm breaking down the barrier between the gut lumen and the blood vessels which drain the bowel.[15]
- HACEK organisms are a group of bacteria that live on the dental gums, and can be seen with IV drug abusers who contaminate their needles with saliva. Patients may also have a history of poor dental hygiene, or pre-existing valvular disease.[16]
Treatment
High dose antibiotics are administered by the intravenous route to maximize diffusion of antibiotic molecules into vegetation(s) from the blood filling the chambers of the heart. This is necessary because neither the heart valves nor the vegetations adherent to them are supplied by blood vessels. Antibiotics are continued for a long time, typically two to six weeks. Specific drug regimens differ depending on the classification of the endocarditis as acute or subacute (acute necessitating treating for S. aureus with oxacillin or vancomycin in addition to gram-negative coverage). Fungal endocarditis requires specific anti-fungal treatment, such as amphotericin B.
In acute endocarditis, due to the fulminant inflammation empirical antibiotic therapy is started immediately after the blood has been drawn for culture. This usually includes oxacillin and gentamicin IV infusions until the culture sensitivity report with the minimum inhibitory concentration comes, when the therapy can be modified to tailor to the microorganism.
In subacute endocarditis, antibiotic treatment is based on the microorganism involved, requiring the culture sensitivity report. So immediate therapy is mainly focused on symptomatic treatment.
The most common organism responsible for infective endocarditis are viridans-group streptococci, which are highly sensitive to penicillin. High dose IV crystalline penicillin every 4hrs for 2 weeks is recommended and still remains the drug of choice. Again it is important to note that antibiotic therapy hinges upon the culture sensitivity report.
Another regimen that is followed for endocarditis is the short course treatment[17] which is a 2 week treatment regimen of benzyl penicillin IV which may be sufficient for S. viridans and S. bovis so long as the following conditions are met:
‣ Endocarditis of a native valve, not on a prosthetic valve
‣ An MIC ≤ 0.1 mg/l
‣ No adverse prognostic factors such as heart failure, aortic valve regurgitation, or conduction defects
‣ No evidence of thromboembolism
‣ No vegetations > 5mm in diameter
‣ Clinical response within 7 days
The short course treatment In patients where the blood culture reveals the causative organism, culture sensitivity reports should be followed to treat the patient, in addition to usage of two bactericidal antibiotics for a minimum of two weeks as a combination therapy.
Surgical debridement of infected material and replacement of the valve with a mechanical or bioprosthetic artificial heart valve is necessary in patients who fail to clear micro-organisms from their blood in response to antibiotic therapy, or in patients who develop cardiac failure resulting from destruction of a valve by infection. Other indications to consider surgery include:[17]
‣ Unstable Prosthetic Valve or Obstruction
‣ Recurrent septic emboli, mycotic aneurysm
‣ Large vegetations
‣ Abscess formation
‣ Early closure of mitral valve
‣ Gram negative species
Infective endocarditis is associated with a 25% mortality.[17]
Non-infective endocarditis
Nonbacterial thrombic endocarditis (NBTE) or marantic endocarditis is most commonly found on previously undamaged valves[2]. As opposed to infective endocarditis, the vegetations in NBTE are small, sterile, and tend to aggregate along the edges of the valve or the cusps[2]. Also unlike infective endocarditis, NBTE does not cause an inflammation response from the body[2]. NBTE usually occurs during a hypercoagulable state such as system wide bacterial infection, or pregnancy, though it is also sometimes seen in patients with venous catheters[2]. NBTE may also occur in patients with cancers, particularly mucinous adenocarcinoma[2].
Typically NBTE does not cause many problems on its own, but parts of the vegetations may break off and embolize to the heart or brain, or they may serve as a focus where bacteria can lodge, thus causing infective endocarditis[2].
Another form of sterile endocarditis, which is fairly rare, is termed Libman-Sacks endocarditis; this form occurs more often in patients with lupus erythematosus and is though to be due to the deposition of immune complexes[2]. Like NBTE, Libman-Sacks endocarditis involves small vegetations, while infective endocarditis is composed of large vegetations[2]. These immune complexes precipitate an inflammation reaction, which helps to differentiate it from NBTE. Also unlike NBTE, Libman-Sacks endocarditis does not seem to have a preferred location of deposition and may form on the undersurfaces of the valves or even on the endocardium[2].
References
- ↑ 1.0 1.1 1.2 Kasper DL, Brunwald E, Fauci AS, Hauser S, Longo DL, Jameson JL (May 2005). Harrison's Principles of Internal Medicine. McGraw-Hill. pp. 731–40. ISBN 0-07-139140-1. OCLC 54501403 56437106 56801936 56967424.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 Mitchell RS, Kumar V, Robbins SL, Abbas AK, Fausto N (2007). Robbins Basic Pathology (8th ed. ed.). Saunders/Elsevier. pp. 406–8. ISBN 1-4160-2973-7.
- ↑ Morris AM (January 2006). "How best to deal with endocarditis". Curr Infect Dis Rep 8 (1): 14–22. doi:. PMID 16448596.
- ↑ Wilson W, Taubert KA, Gewitz M, et al (October 2007). "Prevention of infective endocarditis: guidelines from the American Heart Association". Circulation 116 (15): 1736–54. doi:. PMID 17446442. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=17446442.
- ↑ Durack D, Lukes A, Bright D (1994). "New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service". Am J Med 96 (3): 200–9. doi:. PMID 8154507.
- ↑ 6.0 6.1 Weisse A, Heller D, Schimenti R, Montgomery R, Kapila R (1993). "The febrile parenteral drug user: a prospective study in 121 patients". Am J Med 94 (3): 274–80. doi:. PMID 8452151.
- ↑ 7.0 7.1 Samet J, Shevitz A, Fowle J, Singer D (1990). "Hospitalization decision in febrile intravenous drug users". Am J Med 89 (1): 53–7. doi:. PMID 2368794.
- ↑ 8.0 8.1 8.2 Marantz P, Linzer M, Feiner C, Feinstein S, Kozin A, Friedland G (1987). "Inability to predict diagnosis in febrile intravenous drug abusers". Ann Intern Med 106 (6): 823–8. PMID 3579068.
- ↑ 9.0 9.1 Leibovici L, Cohen O, Wysenbeek A (1990). "Occult bacterial infection in adults with unexplained fever. Validation of a diagnostic index". Arch Intern Med 150 (6): 1270–2. doi:. PMID 2353860.
- ↑ 10.0 10.1 Mellors J, Horwitz R, Harvey M, Horwitz S (1987). "A simple index to identify occult bacterial infection in adults with acute unexplained fever". Arch Intern Med 147 (4): 666–71. doi:. PMID 3827454.
- ↑ Kaech C, Elzi L, Sendi P, et al (2006). "Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre". Clin Microbiol Infect 12 (4): 345–52. doi:. PMID 16524411.
- ↑ Shively B, Gurule F, Roldan C, Leggett J, Schiller N (1991). "Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis". J Am Coll Cardiol 18 (2): 391–7. PMID 1856406.
- ↑ Erbel R, Rohmann S, Drexler M, et al (1988). "Improved diagnostic value of echocardiography in patients with infective endocarditis by transoesophageal approach. A prospective study". Eur Heart J 9 (1): 43–53. PMID 3345769.
- ↑ Topics in Infectious Diseases Newsletter, August 2001, Pseudomonas aeruginosa.
- ↑ Chew SSB, Lubowski DZ (2001). "Clostridium septicum and malignancy".
- ↑ Mirabelle Kelly, MD (June 7, 2005). "HACEK Group Infections".
- ↑ 17.0 17.1 17.2 Hunter JG, Boon NA, Davidson S, Colledge NR, Walker B (2006). Davidson's Principles & Practice of Medicine. Elsevier/Churchill Livingstone. pp. 629–33. ISBN 0-443-10057-8. OCLC 65765046.
External links
- Endocarditis information from Seattle Children's Hospital Heart Center
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||