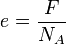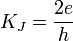Elementary charge
The elementary charge, usually denoted e,[1] is the electric charge carried by a single proton, or equivalently, the negative of the electric charge carried by a single electron. This is a fundamental physical constant.
It has a measured value of approximately 1.602 176 487(40) × 10–19 coulombs[2] In cgs, the value is 4.803 204 27(12) × 10–10 statcoulombs[3] In the system of atomic units as well as some other systems of natural units, e functions as the unit of electric charge, i.e. e = 1 in those unit systems.
The magnitude of the elementary charge was first measured in Robert Millikan's famous oil-drop experiment in 1909.
Contents |
Experimental measurements of the elementary charge
In terms of the Avogadro constant and Faraday constant
If the Avogadro constant NA and the Faraday constant F are independently known, the value of the elementary charge can be deduced, using the formula
(In other words, the charge of one mole of electrons, divided by the number of electrons in a mole, equals the charge of a single electron.)
In practice, this method is not how the most accurate values are measured today. Instead of calculating e from F and NA, in fact NA is calculated from F and e, while e is measured independently by a different method.[4] Nevertheless, this is a legitimate and still quite accurate method, and experimental methodologies are described below:
Measuring the Faraday constant
The value of F can be measured directly using Faraday's laws of electrolysis. Faraday's laws of electrolysis are quantitative relationships based on the electrochemical researches published by Michael Faraday in 1834.[5] In an electrolysis experiment, there is a one-to-one correspondence between the electrons passing through the anode-to-cathode wire and the ions that plate onto or off of the anode or cathode. Measuring the mass change of the anode or cathode, and the total charge passing through the wire (which can be measured as the time-integral of electric current), and also taking into account the molar mass of the ions, one can deduce F.[4]
Measuring the Avogadro constant
The value of the Avogadro constant NA was first approximated by Johann Josef Loschmidt who, in 1865, estimated the average diameter of the molecules in air by a method that is equivalent to calculating the number of particles in a given volume of gas.[6]
Today the value of NA can be measured at very high accuracy by taking an extremely pure crystal (in practice, often silicon), measuring how far apart the atoms are spaced using X-ray diffraction or another method, and accurately measuring the density of the crystal. From this information, one can deduce the weight (in grams) of a single atom; and since the molar mass is known, the number of atoms in a mole can be calculated.[4]
Oil-drop experiment
A famous method for measuring e is Millikan's oil-drop experiment. A small drop of oil in an electric field would move at a rate that balanced the forces of gravity, viscosity (of traveling through the air), and electric force. The forces due to gravity and viscosity could be calculated based on the size and velocity of the oil drop, so electric force could be deduced. Since electric force, in turn, is the product of the electric charge and the known electric field, the electric charge of the oil drop could be accurately computed. By measuring the charges of many different oil drops, it can be seen that the charges are all integer multiples of a single small charge, namely e.
Shot noise
Any electric current will be associated with noise from a variety of sources. One of these sources, shot noise, is due to the fact that the current is not a smooth continual flow, but rather consists of discrete electrons which pass through one at a time. By carefully analyzing the noise of a current, the charge of an electron can be calculated. This method can give a value of e accurate to a few percent.[7]
From the Josephson and von Klitzing constants
Currently, the most accurate method for measuring the elementary charge is by inferring it from measurements of two effects in quantum mechanics: The Josephson effect, voltage oscillations that arise in certain superconducting structures; and the quantum Hall effect, a quantum effect of electrons at low temperatures, strong magnetic fields, and confinement into two dimensions.[8]
The Josephson constant is
(where h is Planck's constant). It can be measured directly using the Josephson effect.
The von Klitzing constant is
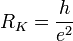 .
.
It can be measured directly using the quantum Hall effect.
From these two constants, the elementary charge can be deduced:
 .
.
Charge quantization
Charge quantization is the statement that every particle or object has a charge which is an integer multiple of the elementary charge e: A charge can be exactly 0, or exactly e, -e, 2e, etc., but not, say, half of e, or -3.8 times e, etc. (This statement must be interpreted carefully; see below for more details.)
This is the reason for the terminology "elementary charge": It is meant to imply that it is an indivisible unit of charge.
Charges less than an elementary charge
There are two known sorts of exceptions to the indivisibility of the elementary charge: quarks and quasiparticles.
- Quarks, first posited in the 1960s, have quantized charge, but the charge is quantized into multiples of 1⁄3 e. However, quarks cannot be seen as isolated particles; they only exist in groupings, and stable groupings of quarks (such as a proton, which consists of three quarks) all have charges that are integer multiples of e. For this reason, either e or 1⁄3 e can be justifiably considered to be "the quantum of charge", depending on the context.
- Quasiparticles are not particles as such, but rather an emergent entity in a complex material system that behaves like a particle. In 1982 Robert Laughlin tried to explain the fractional quantum Hall effect by postulating the existence of fractionally charged quasiparticles. This theory is now widely accepted, but this is not considered to be a violation of the principle of charge quantization, since quasiparticles are not elementary particles.
References
- Fundamentals of Physics, 7th Ed., Halliday, Robert Resnick, and Jearl Walker. Wiley, 2005
- ↑ Note that the symbol e has many other meanings. Most confusingly, in physics, e sometimes denotes the electron charge, i.e. minus the elementary charge.
- ↑ See the NIST posted CODATA value for e.
- ↑ This is derived from the NIST value and uncertainty, using the fact that one coulomb is exactly 2997924580 statcoulombs. (The conversion is ten times the numerical speed of light in meters/second.)
- ↑ 4.0 4.1 4.2 CODATA Recommended Values of the Fundamental Physical Constants: 2006 from CODATA.
- ↑ Ehl, Rosemary Gene; Ihde, Aaron (1954). "Faraday's Electrochemical Laws and the Determination of Equivalent Weights". Journal of Chemical Education 31 (May): 226 – 232.
- ↑ Loschmidt, J. (1865). "Zur Grösse der Luftmoleküle". Sitzungsberichte der kaiserlichen Akademie der Wissenschaften Wien 52 (2): 395–413. English translation.
- ↑ [1]
- ↑ See CODATA 2006. Note that the relative standard uncertainty of e is the same as the uncertainty of the Josephson constant (p95), which in turn agrees with the experimental uncertainty on the Josephson constant (equation (290), page 45).