Battery (electricity)

In electronics, a battery or voltaic cell is a combination of one or more electrochemical Galvanic cells which store chemical energy that can be converted into electric potential energy, creating electricity. Since the invention of the first Voltaic pile in 1800 by Alessandro Volta, the battery has become a common power source for many household and industrial applications, and is now a multi-billion dollar industry.
The name "battery" was coined by Benjamin Franklin for an arrangement of multiple Leyden jars (an early type of capacitor) after a battery of cannon.[1] Common usage has evolved to include a single electrical cell in the definition.[2][3]
Contents[hide] |
History
Although an early form of electrochemical battery called the Baghdad Battery may have been used in antiquity,[4] the modern development of batteries started with the Voltaic pile, invented by the Italian physicist Alessandro Volta in 1800.[5]
In 1791, Luigi Galvani published a report on "animal electricity."[6] He created an electric circuit consisting of two different metals, with one touching a frog's leg and the other touching both the leg and the first metal, thus closing the circuit. In modern terms, the frog's leg served as both the electrolyte and the sensor, and the metals served as electrodes. He noticed that even though the frog was dead, its legs would twitch when he touched them with the metals.
Within a year, Volta realized the frog's moist tissues could be replaced by cardboard soaked in salt water, and the frog's muscular response could be replaced by another form of electrical detection. He already had studied the electrostatic phenomenon of capacitance, which required measurements of electric charge and of electrical potential ("tension"). Building on this experience, Volta was able to detect electric current through his system, also called a Galvanic cell. The terminal voltage of a cell that is not discharging is called its electromotive force (emf), and has the same unit as electrical potential, named (voltage) and measured in volts, in honor of Volta. In 1800, Volta invented the battery by placing many voltaic cells in series, literally piling them one above the other. This Voltaic pile gave a greatly enhanced net emf for the combination,[7] with a voltage of about 50 volts for a 32-cell pile.[8] In many parts of Europe batteries continue to be called piles.[9][10]
Volta did not appreciate that the voltage was due to chemical reactions. He thought that his cells were an inexhaustible source of energy,[11] and that the associated chemical effects (e.g. corrosion) were a mere nuisance, rather than an unavoidable consequence of their operation, as Michael Faraday showed in classic studies that yielded the names anions and cations and anode and cathode (1834).[12] According to Faraday, cations (positively charged ions) are attracted to the cathode, and anions (negatively charged ions) are attracted to the anode.
Although early batteries were of great value for experimental purposes, in practice their voltages fluctuated and they could not provide a large current for a sustained period. Later, starting with the Daniell cell in 1836, batteries provided more reliable currents and were adopted by industry for use in stationary devices, particularly in telegraph networks where they were the only practical source of electricity, since electrical distribution networks did not then exist.[13] These wet cells used liquid electrolytes, which were prone to leakage and spillage if not handled correctly. Many used glass jars to hold their components, which made them fragile. These characteristics made wet cells unsuitable for portable appliances. Near the end of the 19th century, the invention of Dry cell batteries, which replaced liquid in electrolyte with a paste, made portable electrical devices practical.
Since then, batteries have gained popularity as they became portable and useful for a variety of purposes.[14] According to a 2005 estimate, the worldwide battery industry generates US$48 billion in sales each year,[15] with 6% annual growth.[16]
How batteries work

A battery is a device that converts chemical energy directly to electrical energy.[17] It consists of one or more voltaic cells. Each voltaic cell consists of two half cells connected in series by a conductive electrolyte containing anions and cations. One half-cell includes electrolye and the negative electrode (the cathode) and the other includes electrolyte and the positive electrode (the anode). In the redox reaction that powers the battery, reduction (addition of electrons) occurs to cations at the cathode, while oxidation (removal of electrons) occurs to anions the anode.[18] The electrodes do not touch each other but are electrically connected by the electrolyte, which can be either solid or liquid.[19] In many cells, the materials are enclosed in a container, and a separator, which is porous to the electrolyte, which prevents the electrodes from coming into contact.
Each half cell has an electromotive force (or emf), determined by its ability to drive electric current from the interior to the exterior of the cell. The net emf of the battery is the difference between the emfs of its half-cells, as first recognized by Volta.[8] Thus, if the electrodes have emfs 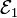 and
and 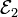 , then the net emf is
, then the net emf is 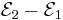 ; in other words, the net emf is difference between the reduction potentials of the half-reactions.[20]
; in other words, the net emf is difference between the reduction potentials of the half-reactions.[20]
The electrical potential difference, or 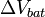 across the terminals of a battery is known as terminal voltage and is measured in volts.[21] The terminal voltage of a battery that is neither charging nor discharging is called the open-circuit voltage and equals the emf of the battery. Because of internal resistance[22], the terminal voltage of a battery that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a battery that is charging exceeds the open-circuit voltage.[23] An ideal battery has negligible internal resistance, so it would maintain a constant terminal voltage of
across the terminals of a battery is known as terminal voltage and is measured in volts.[21] The terminal voltage of a battery that is neither charging nor discharging is called the open-circuit voltage and equals the emf of the battery. Because of internal resistance[22], the terminal voltage of a battery that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a battery that is charging exceeds the open-circuit voltage.[23] An ideal battery has negligible internal resistance, so it would maintain a constant terminal voltage of 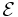 until exhausted, then dropping to zero. If such a battery maintained 1.5 volts and stored a charge of one Coulomb then it would perform 1.5 Joule of work.[21] In actual batteries, the internal resistance increases under discharge,[22] and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a straight line; the shape of the curve varies the chemistry and internal arrangement employed.[24]
until exhausted, then dropping to zero. If such a battery maintained 1.5 volts and stored a charge of one Coulomb then it would perform 1.5 Joule of work.[21] In actual batteries, the internal resistance increases under discharge,[22] and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a straight line; the shape of the curve varies the chemistry and internal arrangement employed.[24]
The voltage developed across a cell's terminals depends on its chemistry: its electrodes and electrolyte. Alkaline and carbon-zinc cells have different chemistries but both measure approximately 1.5 volts, due to the energy release of the associated chemical reactions.[25] However, because of the high electrochemical potential changes in the reactions of lithium compounds, lithium cells can provide as much as 3 volts or more.[26]
Types of batteries

There are many kinds of electrochemical cells, including galvanic cells, electrolytic cells, fuel cells, flow cells and voltaic piles.[27] A battery's characteristics may vary due to many factors including internal chemistry, current drain and temperature.
Batteries are classified into two broad categories, each type with advantages and disadvantages.[28]
- Primary batteries irreversibly (within limits of practicality) transform chemical energy to electrical energy. When the initial supply of reactants is exhausted, energy cannot be readily restored to the battery by electrical means.[29]
- Secondary batteries can be recharged; that is, they can have their chemical reactions reversed by supplying electrical energy to the cell, restoring their original composition.[30]
Historically, some types of primary batteries used, for example, for telegraph circuits, were restored to operation by replacing the components of the battery consumed by the chemical reaction. Secondary batteries are not indefinitely rechargeable due to dissipation of the active materials, loss of electrolyte and internal corrosion.
Primary batteries
Primary batteries can produce current immediately on assembly. Disposable batteries, also called primary cells, are intended to be used once and discarded. These are most commonly used in portable devices that have low current drain, are only used intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available. Primary cells cannot be reliably recharged, since the chemical reactions are not easily reversible and active materials may not return to their original forms. Battery manufacturers recommend against attempting to recharge primary cells.[31]
Common types of disposable batteries include zinc-carbon batteries and alkaline batteries. Generally, these have higher energy densities than rechargeable batteries,[32] but disposable batteries do not fare well under high-drain applications with loads under 75 ohms (75 Ω).[28]
Secondary batteries
It has long been realised that many types of cell chemistries should be able to achieve over 100 Wh/kg at battery level (based on 100% of capacity). One type uses lithium as the negative electrode, a non-aqueous electrolyte, and any of a number of positive electrode materials. Although work on lithium cells has been continuous since the early seventies, successful commercial application of rechargeable lithium cells has been limited by poor cycle life (at most a few hundred cycles) and safety concerns. (In contrast lithium primary cells have found extensive application, although safety concerns limit their domestic use in large capacity versions).
The poor cycle life was due to the difficulty of re-plating lithium onto the negative electrode during recharge. A significant proportion of the lithium re-plated during each cycle is not adequately integrated into the electrode and can no longer be discharged. Hence the loss in capacity. It was long known that this might be overcome by switching from metallic lithium to another material that could 'dissolve' lithium in a solid structure within which the lithium atoms are nevertheless mobile (one of the authors worked on this approach in the late seventies). The problem was that the mass of lithium which could be reversibly dissolved into the materials that were tried was too small compared to the mass of the electrode, negating most of the energy-density advantage one was trying to obtain.
A major breakthrough was achieved by Sony when, in 1991, it introduced cells in which the lithium metal was replaced by a layered-structure carbon electrode into which lithium ions can pass reversibly and in large quantity (roughly one lithium atom per six carbon atoms). This idea had been tried in the past without success, but the breakthrough was in finding the right form of carbon and the necessary pre-treatments for it to function reversibly. The use of carbon in place of lithium metal still does not come without penalties. It reduces the cell potential by about 0.3 volts and adds to the mass of the negative electrode. Fortunately, developments in positive electrode materials had shown how to compensate for this voltage disadvantage and, perhaps still more importantly, opened the way to simpler cell manufacture.
Unlike the negative electrode, positive electrodes have nearly always been 'solid solution electrodes' in which lithium ions are free to move within a layer structure, usually a transition metal oxide or sulphide. The content of lithium ions can be varied over a wide range of composition because the lithium ion charge can be balanced by the variable charge of the transition metal ions. Earlier lithium cells were made in the charged state, i.e. by combining lithium-metal negative electrodes with positive electrodes containing no lithium. As the cell was discharged, the voltage associated with the positive electrode fell as lithium ions were introduced.
The breakthrough in positive electrodes came from a UK Atomic Energy Authority (now AEA Technology) programme, as part of which J. Goodenough at Oxford University devised a new class of positive solid solution electrode materials that were already lithium-containing as synthesised. Combined with the new carbon positive electrodes, this brought two important advantages. Firstly, it enabled cells to be assembled in the discharged state so that it was no longer necessary to handle metallic lithium (or the still very air- and water-sensitive lithium-carbon compound) during manufacture. This greatly simplified the manufacturing process. Secondly, when lithium was removed from the positive electrodes during charge, the voltage associated with the electrode increased, largely compensating the voltage penalty associated with the negative.
Any cells based on lithium-carbon negative electrodes and solid solution positive electrodes are referred to as lithium- carbon or lithium-ion cells. The latter name refers to the fact that the cell can be regarded as a concentration cell in which lithium remains in the form of ions and the voltage is due to the difference in chemical activity between the positive and negative electrodes. The operation of such a cell is shown diagrammatically in Figure 2. They are now under vigorous development by most major battery manufacturers around the world and especially in Japan for use in portable electrical equipment such as computers, power tools and for electric vehicles. In fact, lithium-ion cells are not restricted to graphite as the anode host to the lithium ions, and alternatives are also under development.
Recent developments include batteries with embedded functionality such as USBCELL, with a built-in charger and USB connector within the AA format, enabling the battery to be charged by plugging into a USB port without a charger,[33] and low self-discharge (LSD) mix chemistries such as Hybrio,[34] ReCyko,[35] and Eneloop,[36] where cells are precharged prior to shipping.
Battery capacity and discharging

The more electrolyte and electrode material there is in the cell, the greater the capacity of the cell. Thus a small cell has less capacity than a larger cell, given the same chemistry (e.g. alkaline cells), though they develop the same open-circuit voltage.[37]
Because of the chemical reactions within the cells, the capacity of a battery depends on the discharge conditions such as the magnitude of the current, the duration of the current, the allowable terminal voltage of the battery, temperature and other factors.[37] The available capacity of a battery depends upon the rate at which it is discharged.[38] If a battery is discharged at a relatively high rate, the available capacity will be lower than expected.
The battery capacity that battery manufacturers print on a battery is the product of 20 hours multiplied by the maximum constant current that a new battery can supply for 20 hours at 68 F° (20 C°), down to a predetermined terminal voltage per cell. A battery rated at 100 A·h will deliver 5 A over a 20 hour period at room temperature. However, if it is instead discharged at 50 A, it will run out of charge before the 2 hours as theoretically expected.[39]
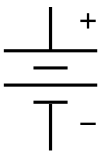
For this reason, a battery capacity rating is always related to an expected discharge duration.
where
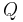 is the battery capacity (typically given in mA·h).
is the battery capacity (typically given in mA·h).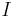 is the current drawn from battery (mA).
is the current drawn from battery (mA).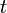 is the amount of time (in hours) that a battery can sustain.
is the amount of time (in hours) that a battery can sustain.
The relationship between current, discharge time, and capacity for a lead acid battery is expressed by Peukert's law. Theoretically, a battery should provide the same amount of energy regardless of the discharge rate, but in real batteries, internal energy losses cause the efficiency of a battery to vary at different discharge rates. When discharging at low rate, the battery's energy is delivered more efficiently than at higher discharge rates.[39]
In general, the higher the ampere-hour rating, the longer the battery will last for a certain load. Installing batteries with different A·h ratings will not affect the operation of a device rated for a specific voltage unless the load limits of the battery are exceeded. Theoretically, a battery would operate at its A·h rating, but realistically, high-drain loads like digital cameras can result in lower actual energy, most notably for alkaline batteries.[28] For example, a battery rated at 2000 mA·h may not sustain a current of 1 A for the full two hours.
Battery lifetime
Life of primary batteries
Even if never taken out of the original package, disposable (or "primary") batteries can lose 8 to 20 percent of their original charge every year at a temperature of about 20°–30°C.[41] This is known as the "self discharge" rate and is due to non-current-producing "side" chemical reactions, which occur within the cell even if no load is applied to it. The rate of the side reactions is reduced if the batteries are stored at low temperature, although some batteries can be damaged by freezing. High or low temperatures may reduce battery performance. This will affect the initial voltage of the battery. For an AA alkaline battery this initial voltage is approximately normally distributed around 1.6 volts.
Diagram  |
Size  |
Capacity (mA·h)  |
Voltage  |
Energy, theoretical (J)  |
ANSI/NEDA  |
IEC  |
Diam. (mm)  |
Mass (g)  |
Height (mm)  |
Length (mm)  |
Width (mm)  |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AAAA | 625 | 1.5 | 3375 | 25A | LR8D425 | 8.3 | 6.5 | 42.5 | cylindrical | cylindrical | |
 |
N | 1000 | 1.5 | 5400 | 910A | LR1 | 12 | 9 | 30.2 | cylindrical | cylindrical |
 |
AAA | 1250 | 1.5 | 6750 | 24A | LR03 | 10.5 | 11.5 | 44.5 | cylindrical | cylindrical |
 |
AA | 2850 | 1.5 | 15390 | 15A | LR6 | 14.5 | 23 | 50.5 | cylindrical | cylindrical |
| J | 625 | 6 | 13500 | 1412A | 4LR61 | prismatic | 30 | 48.5 | 35.6 | 9.18 | |
| 9V | 625 | 9 | 20250 | 1604A | 6LR61 | prismatic | 45.6 | 48.5 | 26.5 | 17.5 | |
 |
C | 8350 | 1.5 | 45090 | 14A | LR14 | 26.2 | 66.2 | 50 | cylindrical | cylindrical |
 |
D | 20500 | 1.5 | 110700 | 13A | LR20 | 34.2 | 148 | 61.5 | cylindrical | cylindrical |
| Lantern | 26000 | 6 | 561600 | 915A | 4R25Y | prismatic | 885 | 112 | 68.2 | 68.2 | |
| Lantern | 26000 | 6 | 561600 | 908A | 4LR25X | prismatic | 885 | 115 | 68.2 | 68.2 | |
| Lantern | 52000 | 6 | 1123200 | 918A | 4LR25-2 | prismatic | 1900 | 127 | 136.5 | 73 |
Discharging performance of all batteries drops at low temperature.[43]
Life of rechargeable batteries
Rechargeable batteries traditionally self-discharge more rapidly than disposable alkaline batteries; up to three percent a day (depending on temperature). However, modern lithium designs have reduced the self-discharge rate to a relatively low level (but still poorer than for primary batteries). Due to their poor shelf life, rechargeable batteries should not be stored and then relied upon to power flashlights or radios in an emergency. For this reason, it is a good idea to keep alkaline batteries on hand. NiCd Batteries are almost always discharged when purchased, and must be charged before first use.
Although rechargeable batteries may be refreshed by charging, they still suffer degradation through usage. Low-capacity nickel metal hydride (NiMH) batteries (1700-2000 mA·h) can be charged for about 1000 cycles, whereas high capacity NiMH batteries (above 2500 mA·h) can be charged for about 500 cycles.[44] Nickel cadmium (NiCd) batteries tend to be rated for 1,000 cycles before their internal resistance increases beyond usable values. Normally a fast charge, rather than a slow overnight charge, will result in a shorter battery lifespan.[44] However, if the overnight charger is not "smart" and cannot detect when the battery is fully charged, then overcharging is likely, which will damage the battery.[45] Degradation usually occurs because electrolyte migrates away from the electrodes or because active material falls off the electrodes. NiCd batteries suffer the drawback that they should be fully discharged before recharge. Without full discharge, crystals may build up on the electrodes, thus decreasing the active surface area and increasing internal resistance. This decreases battery capacity and causes the dreaded "memory effect". These electrode crystals can also penetrate the electrolyte separator, thereby causing shorts. NiMH, although similar in chemistry, does not suffer from "memory effect" to quite this extent.[46] When a battery reaches the end of its lifetime, it will not suddenly lose all of its capacity; rather, its capacity will gradually decrease. [47]
Automotive lead-acid rechargeable batteries have a much harder life. Because of vibration, shock, heat, cold, and sulfation of their lead plates, few automotive batteries last beyond six years of regular use. Automotive starting batteries have many thin plates to provide as much current as possible in a reasonably small package. Typically they are only drained a small amount before recharge. Care should be taken to avoid deep discharging a starting battery, since each charge and discharge cycle causes active material to be shed from the plates. Hole formation in the plates leads to less surface area for the current-producing chemical reactions, resulting in less available current when under load. Leaving a lead-acid battery in a deeply discharged state for any significant length of time allows the lead sulfate to crystallize, making it difficult or impossible to remove during the charging process. This can result in a permanent reduction in the available plate surface, and therefore reduced current output and energy capacity.
"Deep-Cycle" lead-acid batteries such as those used in electric golf carts have much thicker plates to aid their longevity. The main benefit of the lead-acid battery is its low cost; the main drawbacks are its large size and weight for a given capacity and voltage. Lead-acid batteries should never be discharged to below 20% of their full capacity, because internal resistance will cause heat and damage when they are recharged. Deep-cycle lead-acid systems often use a low-charge warning light or a low-charge power cut-off switch to prevent the type of damage that will shorten the battery's life.
Special "reserve" batteries intended for long storage in emergency equipment or munitions keep the electrolyte of the battery separate from the plates until the battery is activated, allowing the cells to be filled with the electrolyte. Shelf times for such batteries can be years or decades. Their construction is costlier than more common forms.
Extending battery life
Battery life can be extended by storing the batteries at a low temperature, as in a refrigerator or freezer, because the chemical reactions in the batteries are slower. Such storage can extend the life of alkaline batteries by ~5%; while the charge of rechargeable batteries can be extended from a few days up to several months.[48] In order to reach their maximum voltage, batteries must be returned to room temperature; discharging an alkaline battery at 250 mAH at 0°C is only half as efficient as it is at 20°C.[32] As a result, alkaline battery manufacturers like Duracell do not recommend refrigerating or freezing batteries.[31]
Battery hazards
A battery explosion is caused by the misuse or malfunction of a battery, such as attempting to recharge a primary (non-rechargeable) battery,[49] or short circuiting a battery.[50] With car batteries, explosions are most likely to occur when a short circuit generates very large currents. In addition, car batteries liberate hydrogen when they are overcharged (because of electrolysis of the water in the electrolyte). Normally the amount of overcharging is very small, as is the amount of explosive gas developed, and the gas dissipates quickly. However, when "jumping" a car battery, the high current can cause the rapid release of large volumes of hydrogen, which can be ignited by a nearby spark (for example, when removing the jumper cables).
When a battery is recharged at an excessive rate, an explosive gas mixture of hydrogen and oxygen may be produced faster than it can escape from within the walls of the battery, leading to pressure build-up and the possibility of the battery case bursting. In extreme cases, the battery acid may spray violently from the casing of the battery and cause injury. Overcharging—that is, attempting to charge a battery beyond its electrical capacity—can also lead to a battery explosion, leakage, or irreversible damage to the battery. It may also cause damage to the charger or device in which the overcharged battery is later used. Additionally, disposing of a battery in fire may cause an explosion as steam builds up within the sealed case of the battery.[50]
The widespread use of batteries has created many environmental concerns, such as toxic metal pollution.[51] Battery manufacture consumes resources and often involves hazardous chemicals. Used batteries also contribute to electronic waste. Some areas now have battery recycling services available to recover some of the materials from used batteries.[52] Batteries may be harmful or fatal if swallowed.[53] Recycling or proper disposal prevents dangerous elements (such as lead, mercury, and cadmium) found in some types of batteries from entering the environment. In the United States, Americans purchase nearly three billion batteries annually, and about 179,000 tons of those end up in landfills across the country.[54] In the United States, the EPA's Mercury-Containing and Rechargeable Battery Management Act of 1996, has reduced the amount of mercury in regular household batteries. Recycling programs for lead and cadmium batteries have been put in place.[55]
Legislation and regulation
European Union
Regulations for banning toxic chemicals and increasing recycling could help drive clean storage technologies. The legislation [56]:
- prohibits the sale of batteries with a weight of 0.0005 percent mercury or 0.002 percent cadmium.
- requires labeling of batteries that contain mercury, cadmium or lead.
- bans the sale of appliances with built-in batteries that can not be replaced.
- requires labeling on batteries to boost recycling rates from about 3 percent now to 25 percent in 2012 and 45 percent in 2016.
See also
- Battery (vacuum tube)
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- Alkaline battery
- Battery Directive
- Battery holder
- Battery isolator
- Battery recycling
- Battery terminals
- Car battery
- Galvanic cell
- Flexible battery
- Electrochemical cell
- Energy density
- Energy storage
- Lead-acid battery
- List of battery sizes
- List of battery types
- Magnetic battery
- Nano titanate
- Nanowire battery
- PP3 battery, also known as "9-volt"
- Printed battery
- Recharging batteries
- Replacing batteries
- Thermal runaway
- Trickle charging
- Watch battery
References
Notes
- ↑ Bellis, Mary. History of the Electric Battery. About.com. Retrieved 11 August 2008.
- ↑ Merriam-Webster Online Dictionary: "battery"
- ↑ "battery" (def. 4b), Merriam-Webster Online Dictionary (2008). Retrieved 6 August 2008.
- ↑ Corder, Gregory W. Using an Unconventional History of the Battery to Engage Students and Explore the Importance of Evidence (PDF). Virginia Journal of Science Education, Vol. 1, No. 1. Retrieved 7 August 2008.
- ↑ Bellis, Mary. Alessandro Volta - Biography of Alessandro Volta - Stored Electricity and the First Battery. About.com. Retrieved 7 August 2008.
- ↑ Bernardi, Walter. The Controversy on Animal Electricity in Eighteenth-Century Italy: Galvani, Volta and Others. Pavia Project Physics. Retrieved 21 May 2008.
- ↑ Weinberg, Willie. Volta: A pioneer in Electrochemistry. The Italian-American Web Site of New York. Retrieved 19 March 2007.
- ↑ 8.0 8.1 Saslow 338.
- ↑ "pila" (def. 1), Pocket Oxford Spanish Dictionary (2005). WordReference.com. Retrieved 6 August 2008.
- ↑ "pile" (def. 2.2), Pocket Oxford-Hachette French Dictionary (2005). WordReference.com. Retrieved 6 August 2008.
- ↑ Stinner, Arthur. Alessandro Volta and Luigi Galvani (PDF). Retrieved 11 August 2008.
- ↑ Electric Battery History - Invention of the Electric Battery. The Great Idea Finder. Retrieved 11 August 2008.
- ↑ Battery History, Technology, Applications and Development. MPower Solutions Ltd. Retrieved 19 March 2007.
- ↑ Batteries: History, Present, and Future of Battery Technology. ExtremeTech. Retrieved 10 September 2007.
- ↑ Power Shift: DFJ on the lookout for more power source investments. Draper Fisher Jurvetson. Retrieved 20 November 2005.
- ↑ Buchmann, Isidor. Battery statistics. Battery University. Retrieved 11 August 2008.
- ↑ "battery" (def. 6), The Random House Dictionary of the English Language, the Unabridged Edition (2nd edition), 1996 ed.
- ↑ Dingrando 665.
- ↑ BBC- Rough Science Library. Retrieved 28 March 2007.
- ↑ Dingrando 666.
- ↑ 21.0 21.1 Knight 943.
- ↑ 22.0 22.1 Knight 976.
- ↑ Terminal Voltage - Tiscali Reference. Originally from Hutchinson Encyclopaedia. Retrieved 7 April 2007.
- ↑ How does the internal battery resistance affect performance?. Battery University. Retrieved 14 August 2008.
- ↑ Dingrando 674.
- ↑ Dingrando 677.
- ↑ "Spotlight on Photovoltaics & Fuel Cells: A Web-based Study & Comparison" (PDF) 1-2. Retrieved on 2007-03-14.
- ↑ 28.0 28.1 28.2 Buchmann, Isidor. Will secondary batteries replace primaries?. Battery University. Retrieved 6 January 2008.
- ↑ Dingrando 675.
- ↑ Fink, Ch. 11, Sec. "Batteries and Fuel Cells."
- ↑ 31.0 31.1 Duracell: Battery Care. Retrieved 10 August 2008.
- ↑ 32.0 32.1 Alkaline Manganese Dioxide Handbook and Application Manual (PDF). Energizer. Retrieved 25 August 2008.
- ↑ USBCELL - Revolutionary rechargeable USB battery that can charge from any USB port. Retrieved 6 November 2007.
- ↑ Long Life Batteries You Can Recharge - Hybrio. Retrieved 6 January 2008.
- ↑ GP ReCyko. Retrieved 6 January 2008.
- ↑ SANYO Presents 'eneloop' : A New Battery in place of Dry Cell Battery for the 21st Century. Retrieved 6 January 2008.
- ↑ 37.0 37.1 Battery Knowledge - AA Portable Power Corp.. Retrieved 16 April 2007.
- ↑ Battery Capacity - Techlib. Retrieved 10 April 2007.
- ↑ 39.0 39.1 Discharge methods. Battery University. Retrieved 14 August 2008.
- ↑ User's Guide to the DS28DG02 (PDF). Maxim Integrated Products. Retrieved 14 August 2008.
- ↑ Self discharge of batteries - Corrosion Doctors. Retrieved 9 September 2007.
- ↑ Alkaline Technical Information. Energizer. Retrieved 11 July 2007.
- ↑ Discharging at high and low temperature
- ↑ 44.0 44.1 Rechargeable battery Tips - NIMH Technology Information. Retrieved 10 August 2007.
- ↑ battery myths vs battery facts - free information to help you learn the difference. Retrieved 10 August 2007.
- ↑ What does ‘memory effect’ mean?. Retrieved 10 August 2007.
- ↑ Battery Life and How To Improve It
- ↑ Ask Yahoo: Does putting batteries in the freezer make them last longer?. Retrieved 7 March 2007.
- ↑ Energizer.com - Learning Center - Energizer and the Environment. Retrieved 17 December 2007.
- ↑ 50.0 50.1 Battery dont's - Global-Batteries. Retrieved 20 August 2007.
- ↑ Batteries - Product Stewardship. EPA. Retrieved 11 September 2007.
- ↑ Battery Recycling » Earth 911. Retrieved 9 September 2007.
- ↑ Product Safety DataSheet - Energizer (PDF, p. 2). Retrieved 9 September 2007.
- ↑ "San Francisco Supervisor Takes Aim at Toxic Battery Waste". Environmental News Network (11 July 2001).
- ↑ Batteries - Municipal Solid Waste. EPA. Retrieved 25 August 2008.
- ↑ http://media.cleantech.com/3441/eu-readies-new-battery-mandates
Further reading
- Dingrando, Laurel; et al. (2007). Chemistry: Matter and Change. New York: Glencoe/McGraw-Hill. ISBN 978-0-07-877237-5. Ch. 21 (pp. 662-695) is on electrochemistry.
- Fink, Donald G.; H. Wayne Beaty (1978). Standard Handbook for Electrical Engineers, Eleventh Edition. New York: McGraw-Hill. ISBN 0-07020974-X.
- Knight, Randall D. (2004). Physics for Scientists and Engineers: A Strategic Approach. San Francisco: Pearson Education. ISBN 0-8053-8960-1. Chs. 28-31 (pp. 879-995) contain information on electric potential.
- Linden, David; Thomas B. Reddy (2001). Handbook Of Batteries. New York: McGraw-Hill. ISBN 0-0713-5978-8.
- Saslow, Wayne M. (2002). Electricity, Magnetism, and Light. Toronto: Thomson Learning. ISBN 0-12-619455-6. Chs. 8-9 (pp. 336-418) have more information on batteries.
External links
- Battery University
- HowStuffWorks: How batteries work
- Comprehensive knowledge base about battery technology, battery applications, chargers and ancillary equipment
|
||||||||||||||||