Diclofenac
 |
|
|
Diclofenac
|
|
| Systematic (IUPAC) name | |
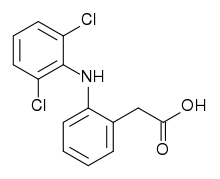
| 2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid | |
| Identifiers | |
| CAS number | |
| ATC code | M01 M02, S01 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C14H11Cl2NO2 |
| Mol. mass | 296.148 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | more than 99% |
| Metabolism | hepatic, no active metabolites exist |
| Half life | 1.2-2 hr (35% of the drug enters enterohepatic recirculation) |
| Excretion | biliary, only 1% in urine |
| Therapeutic considerations | |
| Pregnancy cat. |
A(AU) B (1st. and 2nd. trimenon), X (third trimenon) |
| Legal status |
POM(UK) Rx-only most preparations/countries. Limited OTC some countries. Manufacture and veterinary use is banned in India, Nepal and Pakistan due to imminent extinction of local vultures. |
| Routes | oral, rectal, im, iv (renal- and gallstones), topical |
Diclofenac (marketed as Flector patch, Voltaren, Voltarol, Diclon, Dicloflex Difen, Difene, Cataflam, Pennsaid, Panamor, Rhumalgan, Modifenac, Abitren, Olfen, Voveran, Arthrotec, Dedolor, Deflamat and Zolterol, with various drug dose combinations) is a non-steroidal anti-inflammatory drug (NSAID) taken to reduce inflammation and as an analgesic reducing pain in conditions such as arthritis or acute injury. It can also be used to reduce menstrual pain, dysmenorrhea. The name is derived from its chemical name: 2-(2,6-dichloranilino) phenylacetic acid
In the United Kingdom, India, and the United States, it may be supplied as either the sodium or potassium salt, in China most often as the sodium salt, while in some other countries only as the potassium salt. Diclofenac is available as a generic drug in a number of formulations. Over the counter (OTC) use is approved in some countries for minor aches and pains and fever associated with common infections.
Contents |
Mechanism of action
The action of one single dose is much longer (6 to 8 hours) than the very short half-life that the drug indicates. This could partly be due to a particular high concentration achieved in synovial fluids.
The exact mechanism of action is not entirely known, but it is thought that the primary mechanism responsible for its anti-inflammatory / antipyretic / analgesic action is inhibition of prostaglandin synthesis by inhibition of cyclooxygenase (COX).
Inhibition of COX also decreases prostaglandins in the epithelium of the stomach, making it more sensitive to corrosion by gastric acid. This is also the main side effect of diclofenac. Diclofenac has a low to moderate preference to block the COX2-isoenzyme (approximately 10-fold) and is said to have therefore a somewhat lower incidence of gastrointestinal complaints than noted with indomethacin and aspirin.
Diclofenac may also be a unique member of the NSAIDs. There is some evidence that diclofenac inhibits the lipoxygenase pathways, thus reducing formation of the leukotrienes (also pro-inflammatory autacoids). There is also speculation that diclofenac may inhibit phospholipase A2 as part of its mechanism of action. These additional actions may explain the high potency of diclofenac – it is the most potent NSAID on a broad basis.
There are marked differences among NSAIDs in their selective inhibition of the two subtypes of cyclo-oxygenase, COX-1 and COX-2. Much pharmaceutical drug design has attempted to focus on selective COX-2 inhibition as a way to minimize the gastrointestinal side effects of NSAIDs like aspirin. In practice, use of some COX-2 inhibitors due to their adverse effects has led to massive numbers of patient family lawsuits alleging wrongful death by heart attack, yet other significantly COX-selective NSAIDs like diclofenac have been well-tolerated by most of the population.
Besides the well-known and often cited COX-inhibition, a number of other molecular targets of diclofenac have recently been identified which could contribute to its pain-relieving actions. These include:
- Blockade of voltage-dependent sodium channels (after activation of the channel, diclofenac inhibits its reactivation)
- Blockade of acid-sensing ion channels (ASICs)
- Positive allosteric modulation of KCNQ- and BK-potassium channels (diclofenac opens these channels, leading to hyperpolarization of the cell membrane)
Indications
Diclofenac is used for musculoskeletal complaints, especially arthritis (rheumatoid arthritis, osteoarthritis, spondylarthritis, ankylosing spondylitis), gout attacks, and pain management in case of kidney stones and gallstones. An additional indication is the treatment of acute migraines. Diclofenac is used commonly to treat mild to moderate post-operative or post-traumatic pain, particularly when inflammation is also present, and is effective against menstrual pain.

As long-term use of diclofenac and similar NSAIDs predisposes for peptic ulcer, many patients at risk for this complication are prescribed a combination (Arthrotec) of diclofenac and misoprostol, a synthetic prostaglandin analogue, to protect the gastric mucosa.
An external, gel-based formulation containing 3% of diclofenac (Solareze) is available for the treatment of facial actinic keratosis which is caused by over-exposure to sunlight. Some countries have also approved the external use of diclofenac 1% gel to treat musculoskeletal conditions.
Over-the-counter use against minor aches and pains and fever associated with common infections is also licensed in some countries, such as Australia and New Zealand.
In many countries eye-drops are sold to treat acute and chronic non-bacterial inflammations of the anterior part of the eyes (e.g. postoperative states). A common brand name is Voltaren-ophta.
Off label/investigational uses
Diclofenac is often used to treat chronic pain associated with cancer, particularly if inflammation is also present (Step I of the World Health Organisation (WHO) Scheme for treatment of chronic pain). Good results (sometimes better than those with opioids) have been seen in female breast cancer and in the pain associated with bony metastases. Diclofenac can be combined with opioids if needed. In Europe Combaren exists, a fixed combination of diclofenac and codeine (50 mg each) for cancer treatment. Combinations with psychoactive drugs such as chlorprothixene and/or amitriptyline have also been investigated and found useful in a number of cancer patients.
Fever due to malignant lymphogranulomatosis (Hodgkin's lymphoma) often responds to diclofenac. Treatment can be terminated as soon as the usual treatment with radiation and/or chemotherapy causes remission of fever.
Diclofenac may prevent the development of Alzheimer's disease if given daily in small doses during many years. All investigations were stopped after it was found that some of the other investigated NSAIDs (naproxen, rofecoxib) caused a higher incidence of death cases due to cardiovascular events and stroke compared to placebo.
Diclofenac has been found to increase the blood pressure in patients with Shy-Drager syndrome and Diabetes Mellitus. Currently, this use is highly investigational and cannot be recommended as routine treatment.
Diclofenac has been found to be effective against all strains of multi drug resistant E. coli, with a MIC of 25 microg/mL. Therefore, it may be suggested that diclofenac has the capacity to treat UTI (uncomplicated urinary tract infections) caused by E. coli.[1] It has also been shown to be effective in treating Salmonella infections in mice [2] and is under investigation for the treatment of tuberculosis[3].
Its mechanism of action is not clear yet but it appears to inhibit DNA systhesis. [4]
Contraindications
- Hypersensitivity against diclofenac
- History of allergic reactions (bronchospasm, shock, rhinitis, urticaria) following the use of Aspirin or another NSAID
- Third-trimester pregnancy
- Active stomach and/or duodenal ulceration or gastrointestinal bleeding
- Inflammative intestinal disorders such as Crohn's disease or ulcerative colitis
- Severe insufficiency of the heart (NYHA III/IV)
- Recently, a warning has been issued by FDA not to use to treat patients recovering from heart surgery
- Severe liver insufficiency (Child-Pugh Class C)
- Severe renal insufficiency (creatinine clearance <30 ml/min)
- Caution in patients with preexisting hepatic porphyria, as diclofenac may trigger attacks
- Caution in patients with severe, active bleeding such as cerebral hemorrhage
- NSAIDs in general should be avoided during Dengue Fever.
Side effects
- Diclofenac is among the better tolerated NSAIDs. Though 20% of patients on long-term treatment experience side effects, only 2% have to discontinue the drug, mostly due to gastrointestinal complaints.
Cardiac
- Following the identification of increased risks of heart attacks with the selective COX-2 inhibitor rofecoxib in 2004, attention has focused on all the other members of the NSAIDs group, including Diclofenac. Research results are mixed with a meta-analysis of papers and reports up to April 2006 suggesting a relative increased rate of heart disease of 1.63 compared to non users.[5] Professor Peter Weissberg, Medical Director of the British Heart Foundation said, "However, the increased risk is small and many patients with chronic debilitating pain may well feel that this small risk is worth taking to relieve their symptoms". Only Aspirin was found not to increase the risk of heart disease, however this is known to have a higher rate of gastric ulceration than Diclofenac.
A subsequent large study of 74,838 users of NSAIDs or coxibs, published in May 2006 found no additional cardiovascular risk from Diclofenac use.[6] - Diclofenac has similar COX-2 selectivity to celecoxib [7]. Perhaps related to this selectivity, a review of this constantly changing topic by FDA Medical Officer David Graham concluded in September, 2006 that Diclofenac does increase the risk of myocardial infarction [8].
Gastrointestinal
- Gastrointestinal complaints are most often noted (see above). The development of ulceration and/or bleeding requires immediate termination of treatment with diclofenac. Most patients receive an ulcer-protective drug as prophylaxis during long-term treatment (misoprostol, ranitidine 150mg at bedtime or omeprazole 20 mg at bedtime).
Hepatic
- Liver damage occurs infrequently, and is usually reversible. Hepatitis may occur rarely without any warning symptoms and may be fatal. Patients with osteoarthritis more often develop symptomatic liver disease than patients with rheumatoid arthritis. Liver function should be monitored regularly during long-term treatment. If used for the short term treatment of pain or fever, diclofenac has not been found to be more hepatotoxic than other NSAIDs.
Renal
- Studies in Pakistan showed that diclofenac caused acute kidney failure in vultures when they ate the carcasses of animals that had recently been treated with it (see below at Ecological problems). Species and individual humans that are drug sensitive are initially assumed to lack genes expressing specific drug detoxification enzymes. Because elderly humans have reduced expression levels of all enzymes, elderly human metabolisms may gradually approach those of vultures, thus unexpectedly becoming more diclofenac intolerant.
- NSAIDs "are associated with adverse renal [kidney] effects caused by the reduction in synthesis of renal prostaglandins"[9] in sensitive persons or animal species, and potentially during long term use in non-sensitive persons if resistance to side effects decreases with age. Unfortunately this side effect can't be avoided merely by using a COX-2 selective inhibitor because, "Both isoforms of COX, COX-1 and COX-2, are expressed in the kidney... Consequently, the same precautions regarding renal risk that are followed for nonselective NSAIDs should be used when selective COX-2 inhibitors are administered."[9]
Other
- Bone marrow depression is noted infrequently (leukopenia, agranulocytosis, thrombopenia with/without purpura, aplastic anemia). These conditions may be life-threatening and/or irreversible, if detected too late. All patients should be monitored closely. Diclofenac is a weak and reversible inhibitor of thrombocytic aggregation needed for normal coagulation.
- Diclofenac may disrupt the normal menstrual cycle.
Formulations
Flector Patch is a minimally systemic topical patch formulation of diclofenac. It is indicated for acute pain due to minor sprains, strains, and contusions. The patch has been approved in many other countries outside the U.S.A under different brand names.
Voltaren and Voltarol contain the sodium salt of diclofenac. In the United Kingdom Voltarol can be supplied with either the sodium salt or potassium salt, while Cataflam in some other countries is the potassium salt only.
Diclofenac is available in stomach acid resistant formulations (25 and 50 mg), fast disintegrating oral formulations (50 mg), slow- and controlled-release forms (75, 100 or 150 mg), suppositories (50 and 100 mg), and injectable forms (50 and 75 mg).
Diclofenac is also available over the counter (OTC) in some countries: Voltaren dolo (12.5 mg diclofenac as potassium salt) in Switzerland and Germany, and preparations containing 25 mg diclofenac in New Zealand, and in Sweden (Voltaren T and Diclofenac T 25mg, as the potassium salt).
Diclofenac is also available in Suppository formulation.
NB: Diclofenac is known in the Baltic States as Diclofenacum.
Ecological problems
Use of diclofenac in animals has been reported to have led to a sharp decline in the vulture population in the Indian subcontinent, 95% decline in 2004, 99.9% decline as of 2008.[10] The mechanism is probably renal failure, a known side-effect of diclofenac. Vultures eat the carcasses of domesticated animals that have been administered veterinary diclofenac, and are poisoned by the accumulated chemical. At a meeting of the National Wildlife Board in March 2005, the Government of India announced that it intended to phase out the veterinary use of diclofenac.[11] Meloxicam is a safer (though more expensive) candidate to replace use of diclofenac.[12] "The loss of tens of millions of vultures over the last decade has had major ecological consequences across the Indian subcontinent that pose a potential threat to human health. In many places, populations of feral dogs (Canis familiaris [sic]) have increased sharply from the disappearance of Gyps vultures as the main scavenger of wild and domestic ungulate carcasses. Associated with the rise in dog numbers is an increased risk of rabies"[12] and casualties of almost 50,000 people.[13]
The Government of India cites one of those major consequences as a vulture species extinction.[11] A major shift in transfer of corpse pathogens from vultures to feral dogs and rats can lead to a disease pandemic causing millions of deaths in a crowded country like India.
The loss of vultures has had a social impact on the Indian Zoroastrian Parsi community, who traditionally use vultures to dispose of human corpses in Towers of Silence, but are now compelled to seek alternate methods of disposal.[12]
Diclofenac was shown also to cause harm to freshwater fish species such as rainbow trout[14][15][16][17]
References
- ↑ Mazumdar K, Dutta NK, Dastidar SG, Motohashi N, Shirataki Y (2006). "Diclofenac in the management of E. coli urinary tract infections". In Vivo 20 (5): 613–619. PMID 17091768.
- ↑ Dutta NK, Annadurai S, Mazumdar K, Dastidar SG, Kristiansen JE, Molnar J, Martins M, Amaral L. (2007). "Potential management of resistant microbial infections with a novel non-antibiotic: the anti-inflammatory drug diclofenac sodium". Int. J. Antimicrob. Agents 30 (3): 242–249.
- ↑ Dutta NK, Mazumdar K, Dastidar SG, Park JH (2007). "Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice". Int. J. Antimicrob. Agents 30 (4): 336–340.
- ↑ Dutta NK, Annadurai S, Mazumdar K, Dastidar SG, Kristiansen JE, Molnar J, Martins M, Amaral L (2000). "The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis". Int. J. Antimicrob. Agents 14 (3): 249–51.
- ↑ Kearney P, Baigent C, Godwin J, Halls H, Emberson J, Patrono C (2006). "Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials". BMJ 332 (7553): 1302–8. doi:. PMID 16740558.
- ↑ Solomon D, Avorn J, Stürmer T, Glynn R, Mogun H, Schneeweiss S (2006). "Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk". Arthritis Rheum 54 (5): 1378–89. doi:. PMID 16645966.
- ↑ FitzGerald G, Patrono C (2001). "The coxibs, selective inhibitors of cyclooxygenase-2". N Engl J Med 345 (6): 433–42. doi:. PMID 11496855.
- ↑ Graham D (2006). "COX-2 inhibitors, other NSAIDs, and cardiovascular risk: the seduction of common sense". JAMA 296 (13): 1653–6. doi:. PMID 16968830. http://jama.ama-assn.org/cgi/content/full/296/13/1653.
- ↑ 9.0 9.1 Brater DC (2002). "Renal effects of cyclooxygyenase-2-selective inhibitors". J Pain Symptom Manage 23 (4 Suppl): S15–20; discussion S21–3. doi:. PMID 11992745.
- ↑ Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, Shivaprasad HL, Ahmed S, Chaudhry MJ, Arshad M, Mahmood S, Ali A, Khan AA (2004). "Diclofenac residues as the cause of vulture population decline in Pakistan". Nature 427 (6975): 630–3. doi:. PMID 14745453.
- ↑ 11.0 11.1 Press Information Bureau, Government of India (2005-05-16). "Saving the Vultures from Extiction". Press release. Retrieved on 2006-05-12.
- ↑ 12.0 12.1 12.2 Swan G, Naidoo V, Cuthbert R, Green RE, Pain DJ, Swarup D, Prakash V, Taggart M, Bekker L, Das D, Diekmann J, Diekmann M, Killian E, Meharg A, Patra RC, Saini M, Wolter K (2006). "Removing the threat of diclofenac to critically endangered Asian vultures". PLoS Biol 4 (3): e66. doi:. PMID 16435886.
- ↑ Rabies follows disruption in food cycle
- ↑ Schwaiger et al. (2004). Aquat. Toxicol. 68(2): 141-150
- ↑ Triebskorn et al. (2004). Aquat. Toxicol. 68(2): 151-166
- ↑ Schwaiger & Triebskorn (2005). UBA-Berichte 29/05: 217-226
- ↑ Triebskorn et al. (2007). Analyt. Bioanalyt. Chem. 387(4):1405-1416
External links
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||