Dengue fever
| Dengue virus | ||||||||
|---|---|---|---|---|---|---|---|---|
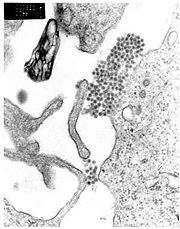 A TEM micrograph showing Dengue virus virions (the cluster of dark dots near the center).
|
||||||||
| Virus classification | ||||||||
|
| Dengue fever Classification and external resources |
||
| ICD-10 | A90. | |
|---|---|---|
| ICD-9 | 061 | |
| DiseasesDB | 3564 | |
| MedlinePlus | 001374 | |
| eMedicine | med/528 | |
| MeSH | C02.782.417.214 | |
Dengue fever (pronounced /ˈdɛŋgeɪ/ (BrE), /ˈdɛŋgiː/ (AmE)) and dengue hemorrhagic fever (DHF) are acute febrile diseases, found in the tropics and Africa, and caused by four closely related virus serotypes of the genus Flavivirus, family Flaviviridae.[1] It is also known as breakbone fever. The geographical spread is similar to malaria, but unlike malaria, dengue is often found in urban areas of tropical nations, including Puerto Rico, Singapore, Malaysia, Taiwan, Thailand, Indonesia, Philippines, Pakistan, India, Brazil, Vietnam, Guyana, Venezuela, Bangladesh and now Samoa[2]. Each serotype is sufficiently different that there is no cross-protection and epidemics caused by multiple serotypes (hyperendemicity) can occur. Dengue is transmitted to humans by the Aedes aegypti or more rarely the Aedes albopictus mosquito, which feed during the day.[3]
Contents |
Signs and symptoms
This is manifested by a sudden onset of severe headache, muscle and joint pains (myalgias and arthralgias—severe pain gives it the name break-bone fever or bonecrusher disease), fever, and rash.[4] The dengue rash is characteristically bright red petechiae and usually appears first on the lower limbs and the chest; in some patients, it spreads to cover most of the body. There may also be gastritis with some combination of associated abdominal pain, nausea, vomiting, or diarrhea.
Some cases develop much milder symptoms which can be misdiagnosed as influenza or other viral infection when no rash is present. Thus travelers from tropical areas may pass on dengue in their home countries inadvertently, having not been properly diagnosed at the height of their illness. Patients with dengue can pass on the infection only through mosquitoes or blood products and only while they are still febrile.
The classic dengue fever lasts about six to seven days, with a smaller peak of fever at the trailing end of the disease (the so-called biphasic pattern). Clinically, the platelet count will drop until the patient's temperature is normal.
Cases of DHF also show higher fever, variable haemorrhagic phenomena, thrombocytopenia, and haemoconcentration. A small proportion of cases lead to dengue shock syndrome (DSS) which has a high mortality rate.
Diagnosis
The diagnosis of dengue is usually made clinically. The classic picture is high fever with no localising source of infection, a petechial rash with thrombocytopenia and relative leukopenia - low platelet and white blood cell count.
The WHO definition of dengue haemorrhagic fever has been in use since 1975; all four criteria must be fulfilled:[5]
- Fever, bladder problem, constant headaches, severe dizziness and loss of appetite.
- Hemorrhagic tendency (positive tourniquet test, spontaneous bruising, bleeding from mucosa, gingiva, injection sites, etc.; vomiting blood, or bloody diarrhea)
- Thrombocytopenia (<100,000 platelets per mm³ or estimated as less than 3 platelets per high power field)
- Evidence of plasma leakage (hematocrit more than 20% higher than expected, or drop in haematocrit of 20% or more from baseline following IV fluid, pleural effusion, ascites, hypoproteinemia)
Dengue shock syndrome is defined as dengue hemorrhagic fever plus:
- Weak rapid pulse,
- Narrow pulse pressure (less than 20 mm Hg)
- Cold, clammy skin and restlessness.
Serology and polymerase chain reaction (PCR) studies are available to confirm the diagnosis of dengue if clinically indicated.
Treatment
The mainstay of treatment is timely supportive therapy to tackle shock due to haemoconcentration and bleeding. Close monitoring of vital signs in critical period (between day 2 to day 7 of fever) is vital. Increased oral fluid intake is recommended to prevent dehydration. Supplementation with intravenous fluids may be necessary to prevent dehydration and significant concentration of the blood if the patient is unable to maintain oral intake. A platelet transfusion is indicated in rare cases if the platelet level drops significantly (below 20,000) or if there is significant bleeding. The presence of melena may indicate internal gastrointestinal bleeding requiring platelet and/or red blood cell transfusion.
Aspirin and non-steroidal anti-inflammatory drugs should be avoided as these drugs may worsen the bleeding tendency associated with some of these infections. Patients may receive paracetamol preparations to deal with these symptoms if dengue is suspected.[6]
Emerging treatments
Emerging evidence suggests that mycophenolic acid and ribavirin inhibit dengue replication. Initial experiments showed a fivefold increase in defective viral RNA production by cells treated with each drug.[7] In vivo studies, however, have not yet been done. Unlike HIV therapy, lack of adequate global interest and funding greatly hampers the development of treatment regime.
Epidemiology

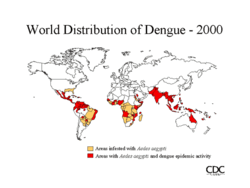
The first epidemics occurred almost simultaneously in Asia, Africa, and North America in the 1780s, shortly after the identification and naming of the disease was identified in 1779. A global pandemic began in Southeast Asia in the 1950s and by 1975 DHF had become a leading cause of death among many children in many countries in that region. Epidemic dengue has become more common since the 1980s. By the late 1990s, dengue was the most important mosquito-borne disease affecting humans after malaria, there being around 40 million cases of dengue fever and several hundred thousand cases of dengue hemorrhagic fever each year. There was a serious outbreak in Rio de Janeiro in February 2002 affecting around one million people and killing sixteen.
On March 20, 2008, the secretary of health of the state of Rio de Janeiro, Sérgio Côrtes, announced that 23,555 cases of dengue, including 30 deaths, had been recorded in the state in less than three months. Côrtes said, "I am treating this as an epidemic because the number of cases is extremely high." Federal Minister of Health, José Gomes Temporão also announced that he was forming a panel to respond to the situation. Cesar Maia, mayor of the city of Rio de Janeiro, denied that there was serious cause for concern, saying that the incidence of cases was in fact declining from a peak at the beginning of February. [8] By April 3, 2008, the number of cases reported rose to 55,000 [9]
Significant outbreaks of dengue fever tend to occur every five or six months. The cyclical rise and fall in numbers of dengue cases is thought to be the result of seasonal cycles interacting with a short-lived cross-immunity for all four strains, in people who have had dengue. When the cross-immunity wears off, the population is then more susceptible to transmission whenever the next seasonal peak occurs. Thus in the longer term of several years, there tend to remain large numbers of susceptible people in the population despite previous outbreaks because there are four different strains of the dengue virus and because of new susceptible individuals entering the target population, either through childbirth or immigration.
There is significant evidence, originally suggested by S.B. Halstead in the 1970s, that dengue hemorrhagic fever is more likely to occur in patients who have secondary infections by another one of dengue fever's four serotypes. One model to explain this process is known as antibody-dependent enhancement (ADE), which allows for increased uptake and virion replication during a secondary infection with a different strain. Through an immunological phenomenon, known as original antigenic sin, the immune system is not able to adequately respond to the stronger infection, and the secondary infection becomes far more serious.[10] This process is also known as superinfection.[11][12]
In Singapore, there are about 4,000–5,000 reported cases of dengue fever or dengue haemorrhagic fever every year. In the year 2003, there were six deaths from dengue shock syndrome. It is believed that the reported cases of dengue are an underrepresentation of all the cases of dengue as it would ignore subclinical cases and cases where the patient did not present for medical treatment. With proper medical treatment, the mortality rate for dengue can therefore be brought down to less than 1 in 1000.
Prevention
Vaccine development
There is no commercially available vaccine for the dengue flavivirus. However, one of the many ongoing vaccine development programs is the Pediatric Dengue Vaccine Initiative which was set up in 2003 with the aim of accelerating the development and introduction of dengue vaccine(s) that are affordable and accessible to poor children in endemic countries.[13] Thai researchers are testing a dengue fever vaccine on 3,000–5,000 human volunteers after having successfully conducted tests on animals and a small group of human volunteers.[14] A number of other vaccine candidates are entering phase I or II testing.[15]
Mosquito control

Primary prevention of dengue mainly resides in mosquito control. There are two primary methods: larval control and adult mosquito control. In urban areas, Aedes mosquitos breed on water collections in artificial containers such as plastic cups, used tires, broken bottles, flower pots, etc. Continued and sustained artificial container reduction or periodic draining of artificial containers is the most effective way of reducing the larva and thereby the Aedes mosquito load in the community. Larvicide treatment is another effective way of control the vector larvae but the larvicide chosen should be long-lasting and preferably have World Health Organization clearance for use in drinking water. There are some very effective insect growth regulators (IGR's) available which are both safe and long-lasting (e.g. pyriproxyfen). For reducing the adult mosquito load, fogging with insecticide is somewhat effective.
Prevention of mosquito bites is another way of preventing disease. This can be achieved either by personal protection or by using mosquito nets.
In 1998, scientists from the Queensland Institute of Research in Australia and Vietnam's Ministry of Health introduced a scheme that encouraged children to place a water bug, the crustacean Mesocyclops, in water tanks and discarded containers where the Aedes aegypti mosquito was known to thrive. This method is viewed as being more cost-effective and more environmentally friendly than pesticides, though not as effective, and requires the ongoing participation of the community.[16]
Potential antiviral approaches
The Dengue virus belong to the familly Flaviviridae, which includes the hepatitis C virus, West Nile and Yellow fever viruses among others. Possible laboratory-based modification of the yellow fever vaccine YF-17D to target the dengue virus via chimeric replacement has been discussed extensively in scientific literature.[17] To date, however, no full scale studies have been conducted.[18]
In 2006, a group of Argentine scientists discovered the molecular replication mechanism of the virus, which could be attacked by disruption of the polymerase's work.[19] In cell culture[20] and murine experiments,[21][22] morpholino antisense oligos have shown specific activity against Dengue virus.
In 2007 replication mechanism of the virus was interrupted by interception of the viral protease,[23] and currently a project to identify new protease interception mechanisms of the whole familly of the virus has been launched.
Etymology
The origins of the word dengue are not clear, but one theory is that it is derived from the Swahili phrase "Ka-dinga pepo", which describes the disease as being caused by an evil spirit.[24] The Swahili word "dinga" may possibly have its origin in the Spanish word "dengue" meaning fastidious or careful, which would describe the gait of a person suffering the bone pain of dengue fever.[25] Alternatively, the use of the Spanish word may derive from the similar-sounding Swahili.[26]
History
The first recorded potential case of dengue fever comes from a Chinese medical encyclopedia from the Chin Dynasty (265–420 AD). The Chinese referred to a “water poison” associated with flying insects. [27] The first definitive case report dates from 1789 and is attributed to Benjamin Rush, who coined the term "breakbone fever" because of the symptoms of myalgia and arthralgia.[28] The viral etiology and the transmission by mosquitoes were deciphered only in the 20th century. Population movements during World War II spread the disease globally.
Use as a biological weapon
Dengue fever was one of more than a dozen agents that the United States researched as potential biological weapons before the nation suspended its biological weapons program.[29]
References
- ↑ "Chapter 4, Prevention of Specific Infectious Diseases". CDC Traveler's Health: Yellow Book. Retrieved on 2007-05-20.
- ↑ http://www.samoalivenews.com/Health/Dengue-Fever-Outbreak-Confirmed-In-Samoa.html
- ↑ Dengue Fever – Information Sheet. World Health Organization, October 9 2006. Retrieved on 2007-11-30.
- ↑ Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. pp. 592. ISBN 0838585299.
- ↑ Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd edition. World Health Organization. Retrieved on 2007-11-30.
- ↑ "Dengue & DHF: Information for Health Care Practitioners". Dengue Fever. CDC Division of Vector-Borne Infectious Diseases (DVBID) (2007-10-22). Retrieved on 2008-10-05.
- ↑ Takhampunya R, Ubol S, Houng HS, Cameron CE, Padmanabhan R (2006). "Inhibition of dengue virus replication by mycophenolic acid and ribavirin". J. Gen. Virol. 87 (Pt 7): 1947–52. doi:. PMID 16760396. http://vir.sgmjournals.org/cgi/content/full/87/7/1947.
- ↑ Fernanda Pontes (20 March 2008), "Secretário estadual de Saúde Sérgio Côrtes admite que estado vive epidemia de dengue" (in Portuguese), O Globo Online, http://oglobo.globo.com/rio/mat/2008/03/20/secretario_estadual_de_saude_sergio_cortes_admite_que_estado_vive_epidemia_de_dengue-426368388.asp.
- ↑ CNN (3 April 2008), "Thousands hit by Brazil outbreak of dengue", CNN, http://www.cnn.com/2008/HEALTH/conditions/04/03/brazil.dengue/index.html.
- ↑ Rothman AL (2004). "Dengue: defining protective versus pathologic immunity". J. Clin. Invest. 113 (7): 946–51. doi:. PMID 15057297. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=379334.
- ↑ Nowak MA, May RM (January 1994). "Superinfection and the evolution of parasite virulence". Proceedings. Biological sciences / the Royal Society 255 (1342): 81–9. doi:. PMID 8153140. http://journals.royalsociety.org/openurl.asp?genre=article&issn=0962-8452&volume=255&issue=1342&spage=81.
- ↑ Levin SA, Pimentel D (1981). "Selection of intermediate rates of increase in parasite-host systems". American Naturalist 117: 308–15.
- ↑ "Pediatric Dengue Vaccine Initiative" (2008). Retrieved on 2008-10-05.
- ↑ "Thailand to test Mahidol-developed dengue vaccine prototype". People's Daily Online (2005-09-05). Retrieved on 2006-10-08.
- ↑ Edelman R (July 2007). "Dengue vaccines approach the finish line". Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 45 Suppl 1: S56–60. doi:. PMID 17582571. http://www.journals.uchicago.edu/doi/abs/10.1086/518148?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ↑ "Water bug aids dengue fever fight", BBC News (2005-02-11). Retrieved on 2008-10-05.
- ↑ Lai CJ, Monath TP (2003). "Chimeric flaviviruses: novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis". Advances in virus research 61: 469–509. PMID 14714441.
- ↑ Querec T, Bennouna S, Alkan S, et al (2006). "Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity". J. Exp. Med. 203 (2): 413–24. doi:. PMID 16461338.
- ↑ Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L, Gamarnik AV (2006). "A 5' RNA element promotes dengue virus RNA synthesis on a circular genome". Genes Dev. 20 (16): 2238–49. doi:. PMID 16882970.
- ↑ Kinney RM, Huang CY, Rose BC, et al (April 2005). "Inhibition of dengue virus serotypes 1 to 4 in vero cell cultures with morpholino oligomers". Journal of virology 79 (8): 5116–28. doi:. PMID 15795296. PMC: 1069583. http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=15795296.
- ↑ Burrer R, Neuman BW, Ting JP, et al (June 2007). "Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models". Journal of virology 81 (11): 5637–48. doi:. PMID 17344287. PMC: 1900280. http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=17344287.
- ↑ Stein DA, Huang CY, Silengo S, et al (September 2008). "Treatment of AG129 mice with antisense morpholino oligomers increases survival time following challenge with dengue 2 virus". The Journal of antimicrobial chemotherapy 62 (3): 555–65. doi:. PMID 18567576. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=18567576.
- ↑ "Project Summary". Discovering Dengue Drug-Together. University of Texas, Medical Branch (2007). Retrieved on 2008-10-05.
- ↑ "Dengue fever: essential data" (1999). Retrieved on 2008-10-05.
- ↑ Harper D (2001). "Etymology: dengue". Online Etymology Dictionary. Retrieved on 2008-10-05.
- ↑ "etymologia: dengue" (PDF). Emerging Infectious Diseases 12 (6): 893. 2006. http://www.cdc.gov/ncidod/eid/vol12no06/pdfs/etymology.pdf.
- ↑ "etymologia: dengue" (PDF). Emerging Infectious Diseases 12 (6): 893. 2006. http://www.cdc.gov/ncidod/eid/vol12no06/pdfs/etymology.pdf.
- ↑ name=Gubler_1998>Gubler DJ (July 1998). "Dengue and dengue hemorrhagic fever". Clinical microbiology reviews 11 (3): 480–96. PMID 9665979. PMC: 88892. http://cmr.asm.org/cgi/pmidlookup?view=long&pmid=9665979.
- ↑ "Chemical and Biological Weapons: Possession and Programs Past and Present", James Martin Center for Nonproliferation Studies, Middlebury College, April 9, 2002, accessed November 14, 2008.
External links
- Dengue fever at the Open Directory Project
- Dengue Virus Genomes database search results from the Dengue Virus Database at the Viral Bioinformatics Resource Center
- Virtual Exhibition about Dengue Fever
|
||||||||||||||||||||||||