Cysteine
- Not to be confused with cystine, its oxidized dimer.
| Cysteine | |
|---|---|
 |
|
 |
 |
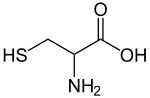
| IUPAC name | (2R)-2-amino-3-sulfanyl-propanoic acid |
| Identifiers | |
| CAS number | 52-90-4 |
| PubChem | |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C3H7NO2S |
| Molar mass | 121.16 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Cysteine (abbreviated as Cys or C)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH2SH. It is a non-essential amino acid, which means that humans can synthesize it. Its codons are UGU and UGC. With a thiol side chain, cysteine is classified as a hydrophilic amino acid. Because of the high reactivity of this thiol, cysteine is an important structural and functional component of many proteins and enzymes. Cysteine is named after cystine, its oxidized dimer.
Contents |
Sources
Dietary sources
Although classified as a non-essential amino acid, in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available. Cysteine is potentially toxic and is catabolized in the gastrointestinal tract and blood plasma. In contrast, cystine travels safely through the GI tract and blood plasma, and is promptly reduced to the two cysteine molecules upon cell entry.
Cysteine is found in most high-protein foods, including:
- Animal sources: pork, sausage meat, chicken, turkey, duck, luncheon meat
- Animal vegetarian sources: eggs, milk, whey protein, ricotta, cottage cheese, yogurt
- Vegan sources: red peppers, garlic, onions, broccoli, brussels sprouts, oats, granola, wheat germ
Industrial sources
- See also Food safety in China#Soy sauce made from human hair.
At the present time, the cheapest source of material from which food-grade L-cysteine may be purified in high yield is by hydrolysis of human hair. Other sources include feathers and pig bristles. The companies producing cysteine by hydrolysis are located mainly in China. There is some debate as to whether or not consuming L-cysteine derived from human hair constitutes cannibalism. Although many other amino acids were accessible via fermentation for some years, L-cysteine was unavailable until 2001 when German company Wacker Chemie introduced a production route via fermentation (non-human, non-animal origin).
Biosynthesis
In animals, biosynthesis begins with the amino acid serine. The sulfur is derived from methionine, which is converted to homocysteine through the intermediate S-adenosylmethionine. Cystathionine beta-synthase then combines homocysteine and serine to form the asymmetrical thioether cystathionine. The enzyme cystathionine gamma-lyase converts the cystathionine into cysteine and alpha-ketobutyrate. In bacteria, cysteine biosynthesis again starts from serine, which is converted to O-acetylserine by the enzyme serine transacetylase. The enzyme O-acetylserine (thiol)-lyase, using sulfide sources, converts this ester into cysteine, releasing acetate.[2]
Biological functions
The cysteine thiol group is nucleophilic and easily oxidized. The reactivity is enhanced when the thiol ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell.[3] Because of its high reactivity, the thiol group of cysteine has numerous biological functions.
Precursor to the antioxidant glutathione
Due to the ability of thiols to undergo redox reactions, cysteine has antioxidant properties. Cysteine's antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans as well as other organisms. The systemic availability of oral glutathione (GSH) is negligible; so it must be biosynthesized from its constituent amino acids, cysteine, glycine, and glutamic acid. Glutamic acid and glycine are readily available in most Western diets, but the availability of cysteine can be the limiting substrate.
Oxidation to cystine linkages
Oxidation of cysteine produces the disulfide cystine. More aggressive oxidants convert cysteine to the corresponding sulfinic acid and sulfonic acid. Cysteine residues play a valuable role by crosslinking proteins, which increases the protein stability in the harsh extracellular environment, and also functions to confer proteolytic resistance (since protein export is a costly process, minimizing its necessity is advantageous). Inside the cell, disulfide bridges between cysteine residues within a polypeptide support the protein's secondary structure. Insulin is an example of a protein with cystine crosslinking, wherein two separate peptide chains are connected by a pair of disulfide bonds.
Protein Disulfide Isomerases catalyze the proper formation of disulfide bonds; the cell transfers dehydroascorbic acid to the endoplasmic reticulum, which oxidises the environment. In this environment, cysteines are, in general, oxidized to cystine and no longer functional as a nucleophiles.
Precursor to iron-sulfur clusters
Cysteine is an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process.[4]
Metal ion binding
Beyond the iron-sulfur proteins, many other metal cofactors in enzymes are bound to the thiolate substituent of cysteinyl residues. Examples include zinc in zinc fingers and alcohol dehydrogenase, copper in the blue copper proteins, iron in cytochrome P450, and nickel in the [NiFe]-hydrogenases.[5] The thiol group also has a high affinity for heavy metals, so that proteins containing cysteine will bind metals such as mercury, lead, and cadmium tightly.[6]
Post-translational modifications
Aside from its oxidation to cystine, cysteine participates in numerous Posttranslational modifications. The nucleophilic thiol group allows cysteine to conjugate to other groups, e.g., in prenylation. Ubiquitin ligases transfer ubiquitin to its pendant, proteins, and caspases, which engage in proteolysis in the apoptotic cycle. Inteins often function with the help of a catalytic cysteine. These roles are typically limited to the intracellular milieu, where the environment is reducing, and cysteine is not oxidized to cystine.
Applications
Cysteine, mainly the L-enantiomer, is a precursor in the food, pharmaceutical, and personal care industries. One of the largest applications is the production of flavors. For example, the reaction of cysteine with sugars in a Maillard reaction yields meat flavors. L-cysteine is also used as a processing aid for baking. Small quantities (in the tens of ppm range) help to soften the dough and thus reduce processing time. http://www.cfsan.fda.gov/~dms/foodic.html
In the field of personal care, cysteine is used for permanent wave applications predominantly in Asia. Again the cysteine is used for breaking up the disulfide bonds in the hair's keratin.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics. Maleimides will selectively attach to cysteine using a covalent Michael addition. Site-directed spin labeling for EPR or paramagnetic relaxation enhanced NMR also uses cysteine extensively.
In a 1994 report released by five top cigarette companies, cysteine is one of the 599 additives to cigarettes. Like most cigarette additives, however, its use or purpose is unknown.[7] Its inclusion in cigarettes could offer two benefits: Acting as an expectorant, since smoking increases mucus production in the lungs; and increasing the beneficial antioxidant glutathione (which is diminished in smokers).
Sheep

Cysteine is required by sheep in order to produce wool: it is an essential amino acid which must be taken in as food from grass. As a consequence, during drought conditions, sheep stop producing wool; however, transgenic sheep which can make their own cysteine have been developed.
Reducing Toxic Effects of Alcohol
Cysteine has been proposed as a preventative or antidote for some of the negative effects of alcohol, including liver damage and hangover. It counteracts the poisonous effects of acetaldehyde[8] , which is the major by-product of alcohol metabolism and is responsible for most of the negative aftereffects and long-term damage associated with alcohol use (but not the immediate effects of drunkenness). Cysteine supports the next step in metabolism, which turns acetaldehyde into the relatively harmless acetic acid. In a rat study, test animals received a LD50 dose of acetaldehyde (the amount which normally kills half of all animals). Those that received cysteine had an 80% survival rate; when thiamine was added, all animals survived.[9] There is not yet direct evidence for or against its effectiveness in humans who consume alcohol at normal levels.
N-acetylcysteine (NAC)
N-acetyl-L-cysteine (NAC) is a derivative of cysteine wherein an acetyl group is attached to the nitrogen atom. This compound is sometimes considered as a dietary supplement, although it is not an ideal source since it is catabolized in the gut. NAC is often used as a cough medicine because it breaks up the disulfide bonds in the mucus and thus liquefies it, making it easier to cough up. NAC is also used as a dietary supplement as already indicated above, as well as a specific antidote in cases of acetaminophen overdose.
See also
- Selenocysteine
- Amino acids
- Thiols
- Cysteine metabolism
- Cystinuria
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved on 2007-05-17.
- ↑ Hell, R. 1997. "Molecular physiology of plant sulfur metabolism" Planta 202:138-148. PMID: 9202491
- ↑ Bulaj G, Kortemme T, Goldenberg D (1998). "Ionization-reactivity relationships for cysteine thiols in polypeptides.". Biochemistry 37 (25): 8965–72. doi:. PMID 9636038.
- ↑ Roland Lill, Ulrich Mühlenhoff “Iron-Sulfur Protein Biogenesis in Eukaryotes: Components and Mechanisms” Annual Review of Cell and Developmental Biology, 2006, Volume 22, pp. 457-486. doi:10.1146/annurev.cellbio.22.010305.104538.
- ↑ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ↑ Baker D, Czarnecki-Maulden G (1987). "Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities.". J Nutr 117 (6): 1003–10. doi:10.1126/science.2237411.<br> (inactive 2008-06-25). PMID 3298579.
- ↑ http://quitsmoking.about.com/cs/nicotineinhaler/a/cigingredients.htm
- ↑ http://www.lef.org/protocols/prtcl-004.shtml
- ↑ Effects of cysteine on acetaldehyde lethality http://www.springerlink.com/content/w307w62037125v33/
External links
- Computational Chemistry Wiki
- International Kidney Stone Institute
- http://www.chemie.fu-berlin.de/chemistry/bio/aminoacid/cystein en.html
- On the hydrophobic nature of cysteine.
- 952-10-3056-9 Interaction of alcohol and smoking in the pathogenesis of upper digestive tract cancers - possible chemoprevention with cysteine
- Cystine Kidney Stones
|
||||||||||||||
|
|||||