Crohn's disease
| Crohn's disease Classification and external resources |
|
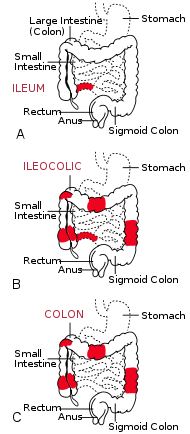 |
|
|---|---|
| The three most common sites of intestinal involvement in Crohn's disease are
ileal, ileocolic and colonic.[1] |
|
| ICD-10 | K50. |
| ICD-9 | 555 |
| OMIM | 266600 |
| DiseasesDB | 3178 |
| MedlinePlus | 000249 |
| eMedicine | med/477 ped/507 radio/197 |
| MeSH | D003424 |
Crohn's disease (also known as granulomatous colitis and regional enteritis) is an inflammatory disease of the digestive system which may affect any part of the gastrointestinal tract from mouth to anus. As a result, the symptoms of Crohn's disease can vary significantly among afflicted individuals. The main gastrointestinal symptoms are abdominal pain, diarrhea (which may be visibly bloody), vomiting, or weight loss.[2][3] Crohn's disease can also cause complications outside of the gastrointestinal tract such as skin rashes, arthritis, and inflammation of the eye.[1]
The precise cause of Crohn's disease is not known. The disease occurs when the immune system attacks the gastrointestinal tract and for this reason, Crohn's disease is considered an autoimmune disease. This autoimmune activity produces inflammation in the gastrointestinal tract, and therefore Crohn's disease is classified as an inflammatory bowel disease.
Like many other autoimmune diseases, Crohn's disease is believed to be genetically linked. The highest risk occurs in individuals with siblings who have the disease. Males and females are equally affected. Smokers are three times more likely to develop Crohn's disease.[4] Crohn's disease affects between 400,000 and 600,000 people in North America.[5] Prevalence estimates for Northern Europe have ranged from 27–48 per 100,000.[6] Crohn's disease tends to present initially in the teens and twenties, with another peak incidence in the fifties to seventies, although the disease can occur at any age.[1][7]
Unlike the other major types of inflammatory bowel disease, there is no known drug based or surgical cure for Crohn's disease.[8] Treatment options are restricted to controlling symptoms, putting and keeping the disease in remission and preventing relapse.
The disease was independently described in 1904 by Polish surgeon Antoni Leśniowski and in 1932 by American gastroenterologist Burrill Bernard Crohn, for whom the disease was named. Crohn, along with two colleagues, described a series of patients with inflammation of the terminal ileum, the area most commonly affected by the illness.[9] For this reason, the disease has also been called regional ileitis[9] or regional enteritis.
Contents |
Classification

Crohn's disease is generally classified as an autoimmune disease. It invariably affects the gastrointestinal tract and most gastroenterologists categorize the presenting disease by the affected areas. Ileocolic Crohn's disease, which affects both the ileum (the last part of the small intestine that connects to the large intestine) and the large intestine, accounts for fifty percent of cases. Crohn's ileitis, affecting the ileum only, accounts for thirty percent of cases, and Crohn's colitis, affecting the large intestine, accounts for the remaining twenty percent of cases, and may be particularly difficult to distinguish from ulcerative colitis. The disease can attack any part of the digestive tract, from mouth to anus. However, individuals affected by the disease rarely fall outside these three classifications, being affected in other parts of the gastrointestinal tract such as the stomach and esophagus.[1] Crohn's disease may also be categorized by the behaviour of disease as it progresses. This was formalized in the Vienna classification of Crohn's disease.[10] There are three categories of disease presentation in Crohn's disease: stricturing, penetrating, and inflammatory. Stricturing disease causes narrowing of the bowel which may lead to bowel obstruction or changes in the caliber of the feces. Penetrating disease creates abnormal passageways (fistulae) between the bowel and other structures such as the skin. Inflammatory disease (or non-stricturing, non-penetrating disease) causes inflammation without causing strictures or fistulae.[10][11]
Symptoms

Many people with Crohn's disease have symptoms for years prior to the diagnosis.[12] The usual onset is between 15 and 30 years of age but can occur at any age.[13] Because of the 'patchy' nature of the gastrointestinal disease and the depth of tissue involvement, initial symptoms can be more vague than with ulcerative colitis. People with Crohn's disease will go through periods of flare-ups and remission.
Gastrointestinal symptoms
Abdominal pain may be the initial symptom of Crohn's disease. The pain is commonly cramp-like and is not relieved by defecation. It is often accompanied by diarrhea, which may or may not be bloody, though diarrhea is not uncommon especially in those who have had surgery. People who have had surgery or multiple surgeries often end up with short bowel syndrome of the gastrointestinal tract. The nature of the diarrhea in Crohn's disease depends on the part of the small intestine or colon that is involved. Ileitis typically results in large-volume watery feces. Colitis may result in a smaller volume of feces of higher frequency. Fecal consistency may range from solid to watery. In severe cases, an individual may have more than 20 bowel movements per day and may need to awaken at night to defecate.[1][7][14][15] Visible bleeding in the feces is less common in Crohn's disease than in ulcerative colitis, but may be seen in the setting of Crohn's colitis.[1] Bloody bowel movements are typically intermittent, and may be bright or dark red in colour. In the setting of severe Crohn's colitis, bleeding may be copious.[7] Flatulence and bloating may also add to the intestinal discomfort.[7]
Symptoms caused by intestinal stenosis are also common in Crohn's disease. Abdominal pain is often most severe in areas of the bowel with stenoses. In the setting of severe stenosis, vomiting and nausea may indicate the beginnings of small bowel obstruction.[7] Crohn's disease may also be associated with primary sclerosing cholangitis, a type of inflammation of the bile ducts.
Perianal discomfort may also be prominent in Crohn's disease. Itchiness or pain around the anus may be suggestive of inflammation, fistulization or abscess around the anal area[1] or anal fissure. Perianal skin tags are also common in Crohn's disease.[16] Fecal incontinence may accompany peri-anal Crohn's disease. At the opposite end of the gastrointestinal tract, the mouth may be affected by non-healing sores (aphthous ulcers). Rarely, the esophagus, and stomach may be involved in Crohn's disease. These can cause symptoms including difficulty swallowing (odynophagia), upper abdominal pain, and vomiting.[17]
Systemic symptoms
Crohn's disease, like many other chronic, inflammatory diseases, can cause a variety of systemic symptoms.[1] Among children, growth failure is common. Many children are first diagnosed with Crohn's disease based on inability to maintain growth.[18] As Crohn's disease may manifest at the time of the growth spurt in puberty, up to 30% of children with Crohn's disease may have retardation of growth.[19] Fever may also be present, though fevers greater than 38.5 ˚C (101.3 ˚F) are uncommon unless there is a complication such as an abscess[1] Among older individuals, Crohn's disease may manifest as weight loss. This is usually related to decreased food intake, since individuals with intestinal symptoms from Crohn's disease often feel better when they do not eat and might lose their appetite.[18] People with extensive small intestine disease may also have malabsorption of carbohydrates or lipids, which can further exacerbate weight loss.[20]
Extraintestinal symptoms
In addition to systemic and gastrointestinal involvement, Crohn's disease can affect many other organ systems.[21] Inflammation of the interior portion of the eye, known as uveitis, can cause eye pain, especially when exposed to light (photophobia). Inflammation may also involve the white part of the eye (sclera), a condition called episcleritis. Both episcleritis and uveitis can lead to loss of vision if untreated.
Crohn's disease is associated with a type of rheumatologic disease known as seronegative spondyloarthropathy. This group of diseases is characterized by inflammation of one or more joints (arthritis) or muscle insertions (enthesitis). The arthritis can affect larger joints such as the knee or shoulder or may exclusively involve the small joints of the hand and feet. The arthritis may also involve the spine, leading to ankylosing spondylitis if the entire spine is involved or simply sacroiliitis if only the lower spine is involved. The symptoms of arthritis include painful, warm, swollen, stiff joints and loss of joint mobility or function.
Crohn's disease may also involve the skin, blood, and endocrine system. One type of skin manifestation, erythema nodosum, presents as red nodules usually appearing on the shins. Erythema nodosum is due to inflammation of the underlying subcutaneous tissue and is characterized by septal panniculitis. Another skin lesion, pyoderma gangrenosum, is typically a painful ulcerating nodule. Crohn's disease also increases the risk of blood clots; painful swelling of the lower legs can be a sign of deep venous thrombosis, while difficulty breathing may be a result of pulmonary embolism. Autoimmune hemolytic anemia, a condition in which the immune system attacks the red blood cells, is also more common in Crohn's disease and may cause fatigue, pallor, and other symptoms common in anemia. Clubbing, a deformity of the ends of the fingers, may also be a result of Crohn's disease. Finally, Crohn's disease may cause osteoporosis, or thinning of the bones. Individuals with osteoporosis are at increased risk of bone fractures.[6]
Crohn's disease can also cause neurological complications (reportedly in up to 15% of patients).[22] The most common of these are seizures, stroke, myopathy, peripheral neuropathy, headache and depression.[22]
Crohn's patients often also have issues with Small bowel bacterial overgrowth syndrome, which has similar symptoms.[23]
Complications

Crohn's disease can lead to several mechanical complications within the intestines, including obstruction, fistulae, and abscesses. Obstruction typically occurs from strictures or adhesions which narrow the lumen, blocking the passage of the intestinal contents. Fistulae can develop between two loops of bowel, between the bowel and bladder, between the bowel and vagina, and between the bowel and skin. Abscesses are walled off collections of infection, which can occur in the abdomen or in the perianal area in Crohn's disease sufferers.
Crohn's disease also increases the risk of cancer in the area of inflammation. For example, individuals with Crohn's disease involving the small bowel are at higher risk for small intestinal cancer. Similarly, people with Crohn's colitis have a relative risk of 5.6 for developing colon cancer.[24] Screening for colon cancer with colonoscopy is recommended for anyone who has had Crohn's colitis for eight years, or more.[25] Some study suggest that there is a role for chimioprotection in the prevention of colorectal cancer in Crohn's involving the colon; two agents have been suggested, folate and mesalamine preparations.[26]
Individuals with Crohn's disease are at risk of malnutrition for many reasons, including decreased food intake and malabsorption. The risk increases following resection of the small bowel. Such individuals may require oral supplements to increase their caloric intake, or in severe cases, total parenteral nutrition (TPN). Most people with moderate or severe Crohn's disease are referred to a dietitian for assistance in nutrition.[27]
Crohn's disease can cause significant complications including bowel obstruction, abscesses, free perforation and hemorrhage.[28]
Crohn's disease can be problematic during pregnancy, and some medications can cause adverse outcomes for the fetus or mother. Consultation with an obstetrician and gastroenterologist about Crohn's disease and all medications allows preventative measures to be taken. In some cases, remission can occur during pregnancy. Certain medications can also impact sperm count or may otherwise adversely affect a man's ability to conceive.[29]
Cause

The exact cause of Crohn's disease is unknown. However, environmental and genetic factors have been invoked in the pathogenesis of the disease. Research has indicated that Crohn's disease has a strong genetic link.[30] The disease runs in families and those with a sibling with the disease are 30 times more likely to develop it than the normal population. Ethnic background is also a risk factor. Until very recently, whites and European Jews accounted for the vast majority of the cases in the United States, and in most industrialized countries, this demographic is still true. However, the European commission makes an interesting point:
A review of the literature suggests that Crohn’s disease is more prevalent in Western populations with northern European and Anglo-Saxon ethnic derivation, than in populations of southern Europe, Asia and Africa. However, when the Asian people migrate to urban-industrial societies of the West, they become as susceptible to the disease as the population of their host countries, suggesting environmental factors in the aetiology of Crohn’s disease143.[31]
Mutations in the CARD15 gene (also known as the NOD2 gene) are associated with Crohn's disease[32] and with susceptibility to certain phenotypes of disease location and activity.[33] In earlier studies, only two genes were linked to Crohn's, but scientists now believe there are over eight genes that show genetics play a role in the disease, either directly through causation or indirectly as with a mediator variable. Anomalies in the XBP1 gene have recently been identified as a factor.[34]
A handful of cases of Crohn's disease cases were reported at the turn of the 20th century, but since then, the disease has continued to increase in prevalence dramatically. Some argue that this increase has been the result of a genetic shift in the population caused by conditions favoring individuals carrying the genes linked with the disease. These conditions could be a lower infant mortality rate or better health care in the nations that have the highest incidence of disease (industrialized nations). Another explanation is that modern industrial practices have given rise to increased disease prevalence via infectious diseases. A common recurrent theory is that a specific species of Mycobacterium, Mycobacterium avium subspecies paratuberculosis, is responsible for both Johne's disease and Crohn's disease, and modern industrial farming practices have led to the spread of Mycobacterium avium subspecies paratuberculosis.[31]
Some studies have suggested that Crohn's disease, ulcerative colitis and irritable bowel syndrome have the same underlying cause. Biopsy samples taken from the colons of all three patient groups were found to produce elevated levels of a serine protease. [35] Experimental introduction of the serine protease into mice has been found to produce widespread pain associated with irritable bowel syndrome as well as colitis, which is associated with all three diseases.[36] The authors of that study were unable to identify the source of the protease, but a separate review noted that regional and temporal variations in those illnesses follow those associated with infection with a poorly understood protozoan, Blastocystis. [37]
Others argue that Crohn's disease is caused by a combination of environmental and genetic factors. Many environmental factors have also been hypothesized as causes or risk factors for Crohn's disease. Proven environmental risk factors include living in an industrialized country, smoking, and living in an urban area. Diets high in sweet, fatty or refined foods may also play a role. A retrospective Japanese study found that those diagnosed with Crohn's disease had higher intakes of sugar, fat, fish and shellfish than controls prior to diagnosis.[38] A similar study in Israel also found higher intakes of fats (especially chemically modified fats) and sucrose, with lower intakes of fructose and fruits, water, potassium, magnesium and vitamin C in the diets of Crohn's disease sufferers before diagnosis,[39] and cites three large European studies in which sugar intake was significantly increased in people with Crohn's disease compared with controls. The most common forms of microparticles include titanium dioxide, aluminosilicates, anatase, calcium phosphate, and soil residue. These substances are ubiquitous in processed food and most toothpastes and lip glosses. Soil residue is found on fresh fruits and vegetables unless carefully removed.
Smoking has been shown to increase the risk of the return of active disease, or "flares".[40] The introduction of hormonal contraception in the United States in the 1960s is linked with a dramatic increase in the incidence rate of Crohn's disease. Although a causal linkage has not been effectively shown, there remain fears that these drugs work on the digestive system in similar ways to smoking.[41]
Additionally, many in the scientific community believe that early childhood exposure to illness is necessary to the creation of a proper immune system for those with the genetic susceptibility for Crohn's Disease. Higher incidences of Crohn's Disease are associated with cleaner living conditions. Throughout the early and mid-20th century in the United States, the disease was strongly associated with upper-class populations, and today the disease does not yet exist in the many Third World countries, despite the fact that it occurs in all races. CD is also associated with first born and single children (because they would have less exposure to childhood illness from siblings) and in populations that have low incidences of gastric cancer. Gastric cancer is most often caused by the bacterium Helicobacter pylori that flourishes in cramped and unsanitary conditions.[42]
Abnormalities in the immune system have often been invoked as being causes of Crohn's disease. Crohn's disease is thought to be an autoimmune disease, with inflammation stimulated by an over-active Th1 cytokine response.[43] However, more recent evidence has shown that Th17 is of greater importance in the disease.[44] The most recent gene to be implicated in Crohn's disease is ATG16L1, which may reduce the effectiveness of autophagy, and hinder the body's ability to attack invasive bacteria.[45]
A variety of pathogenic bacteria were initially suspected of being causative agents of Crohn's disease and many groups still suspect Mycobacterium avium subspecies paratuberculosis.[46] However, the consensus of many health care professionals for several years has been that a variety of microorganisms are simply taking advantage of their host's weakened mucosal layer and inability to clear bacteria from the intestinal walls, both symptoms of the disease.[47] Some studies have linked Mycobacterium avium subsp. paratuberculosis to Crohn's disease, in part because it causes a very similar disease, Johne's disease, in cattle.[48] The mannose bearing antigens, mannins, from yeast may also elicit pathogenic anti saccharomyces cerevisiae antibodies.[49] Studies have linked specific strains of enteroadherent E. coli to the disease. Still this relationship and evidence of contributions by other species remains controversial.[31][50][51][52] And more recently a relationship between MAP and E. coli has been uncovered. Scientists at the university of Liverpool have discovered that "Mycobacteria release a complex molecule containing a sugar, called mannose. This molecule prevents a type of white blood cells, called macrophages, from killing internalized E.Coli.”[53]
Some scientific studies and court rulings have posited that Accutane is a probable cause of IBD, a group of diseases including Crohn's Disease and Ulcerative colitis, in some individuals. Three cases in the United States have gone to trial thus far, with all three resulting in multi-million dollar judgments against the makers of isotretinoin; there are an additional 425 cases pending. [54] [55] [56] [57] [58]
Pathophysiology

At the time of colonoscopy, biopsies of the colon are often taken in order to confirm the diagnosis. There are certain characteristic features of the pathology seen that point toward Crohn's disease. Crohn's disease shows a transmural pattern of inflammation, meaning that the inflammation may span the entire depth of the intestinal wall.[1] Grossly, ulceration is an outcome seen in highly active disease. There is usually an abrupt transition between unaffected tissue and the ulcer. Under a microscope, biopsies of the affected colon may show mucosal inflammation. This inflammation is characterized by focal infiltration of neutrophils, a type of inflammatory cell, into the epithelium. This typically occurs in the area overlying lymphoid aggregates. These neutrophils, along with mononuclear cells, may infiltrate into the crypts leading to inflammation (crypititis) or abscess (crypt abscess). Granulomas, aggregates of macrophage derivatives known as giant cells, are found in 50% of cases and are most specific for Crohn's disease. The granulomas of Crohn's disease do not show "caseation", a cheese-like appearance on microscopic examination that is characteristic of granulomas associated with infections such as tuberculosis. Biopsies may also show chronic mucosal damage as evidenced by blunting of the intestinal villi, atypical branching of the crypts, and change in the tissue type (metaplasia). One example of such metaplasia, Paneth cell metaplasia, involves development of Paneth cells (typically found in the small intestine) in other parts of the gastrointestinal system.[59]
According to the volume 1 of the 10th Edition of Brunner and Suddarth’s Textbook of Medical-Surgical Nursing Regional enteritis is a subacute and chronic inflammation that extends through the layers (ie, transmural lesion) of the bowel wall from the intestinal mucosa. It is characterized by periods of remissions and exacerbations. The disease process begins with edema and thickening of the mucosa. Ulcers begin to appear on the inflamed mucosa. These lesions are not in continuous contact with one another and are separated by normal tissue. Fistulas, fissures, and abscesses form as the inflammation extends into the peritoneum. Granulomas occur in one half of patients. In advanced cases, the intestinal mucosa has a cobblestone appearance. As the disease advances, the bowel wall thickens and becomes fibrotic, and the intestinal lumen narrows. Diseased bowel loops sometimes adhere to other loops surrounding them.
Diagnosis

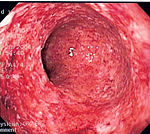
The diagnosis of Crohn's disease can sometimes be challenging,[12] and a number of tests are often required to assist the physician in making the diagnosis.[7] Even with a full battery of tests it may not be possible to diagnose Crohn's with complete certainty; a colonoscopy is approximately 70% effective in diagnosing the disease with further being less effective. Disease in the small bowel is particularly difficult to diagnose as a traditional colonoscopy only allows access to the colon and lower portions of the small intestines; introduction of the Capsule endoscopy[60] aids in endoscopic diagnosis.
Endoscopy
A colonoscopy is the best test for making the diagnosis of Crohn's disease as it allows direct visualization of the colon and the terminal ileum, identifying the pattern of disease involvement. Occasionally, the colonoscope can travel past the terminal ileum but it varies from patient to patient. During the procedure, the gastroenterologist can also perform a biopsy, taking small samples of tissue for laboratory analysis which may help confirm a diagnosis. As 30% of Crohn's disease involves only the ileum,[1] cannulation of the terminal ileum is required in making the diagnosis. Finding a patchy distribution of disease, with involvement of the colon or ileum but not the rectum, is suggestive of Crohn's disease, as are other endoscopic stigmata.[61]The utility of capsule endoscopy for this, however, is still uncertain.[62]
Radiologic tests
A small bowel follow-through may suggest the diagnosis of Crohn's disease and is useful when the disease involves only the small intestine. Because colonoscopy and gastroscopy allow direct visualization of only the terminal ileum and beginning of the duodenum, they cannot be used to evaluate the remainder of the small intestine. As a result, a barium follow-through x-ray, wherein barium sulfate suspension is ingested and fluoroscopic images of the bowel are taken over time, is useful for looking for inflammation and narrowing of the small bowel.[61][63] Barium enemas, in which barium is inserted into the rectum and fluoroscopy used to image the bowel, are rarely used in the work-up of Crohn's disease due to the advent of colonoscopy. They remain useful for identifying anatomical abnormalities when strictures of the colon are too small for a colonoscope to pass through, or in the detection of colonic fistulae.[64]
CT and MRI scans are useful for evaluating the small bowel with enteroclysis protocols.[65]They are additionally useful for looking for intra-abdominal complications of Crohn's disease such as abscesses, small bowel obstruction, or fistulae.[66] Magnetic resonance imaging (MRI) are another option for imaging the small bowel as well as looking for complications, though it is more expensive and less readily available[67]
Blood tests
A complete blood count may reveal anemia, which may be caused either by blood loss or vitamin B12 deficiency. The latter may be seen with ileitis because vitamin B12 is absorbed in the ileum.[68] Erythrocyte sedimentation rate, or ESR, and C-reactive protein measurements can also be useful to gauge the degree of inflammation.[69] It is also true in patient with ilectomy done in response to the complication. Another cause of anaemia is anaemia of chronic disease, characterized by its microcytic and hypochromic anaemia. There can be various reasons for anaemia, including medication used in treatment of inflammatory bowel disease like azathioprine which can lead to cytopenia and sulfasalazine which can also result in folate malabsorption, etc. Testing for anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (ANCA) has been evaluated to identify inflammatory diseases of the intestine[70] and to differentiate Crohn's disease from ulcerative colitis.[71]
Feces
Calprotectin is a protein found in neutrophil cytosol. Neutrophils are integral to the inflammatory process caused by Crohn's disease. Faecal Calprotectin is a protein measured in a patient's feces. The fecal concentration correlates well with inflammation and disease activity. Normal fecal calprotectin level in a patient with active gastrointestinal symptoms excludes inflammatory bowel disease as a likely diagnosis and in many cases negates the need for colonoscopy or radio labelled white cell scanning[72].
Comparison with ulcerative colitis
The most common disease that mimics the symptoms of Crohn's disease is ulcerative colitis, as both are inflammatory bowel diseases that can affect the colon with similar symptoms. It is important to differentiate these diseases, since the course of the diseases and treatments may be different. In some cases, however, it may not be possible to tell the difference, in which case the disease is classified as indeterminate colitis.[14][1][7]
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Terminal ileum involvement | Commonly | Seldom |
| Colon involvement | Usually | Always |
| Rectum involvement | Seldom | Usually[73] |
| Involvement around the anus | Common[74] | Seldom |
| Bile duct involvement | No increase in rate of primary sclerosing cholangitis | Higher rate[75] |
| Distribution of Disease | Patchy areas of inflammation (Skip lesions) | Continuous area of inflammation[73] |
| Endoscopy | Deep geographic and serpiginous (snake-like) ulcers | Continuous ulcer |
| Depth of inflammation | May be transmural, deep into tissues[74][1] | Shallow, mucosal |
| Fistulae | Common[74] | Seldom |
| Stenosis | Common | Seldom |
| Autoimmune disease | Widely regarded as an autoimmune disease | No consensus |
| Cytokine response | Associated with Th17 [44] | Vaguely associated with Th2 |
| Granulomas on biopsy | Can have granulomas[74] | Granulomas not seen[73] |
| Surgical cure | Often returns following removal of affected part | Usually cured by removal of colon |
| Smoking | Higher risk for smokers | Lower risk for smokers[73] |
Treatment
Currently there is no cure for Crohn's disease and remission may not be possible or prolonged if achieved;[76] in cases where remission is possible, relapse can be prevented and symptoms controlled with medication, lifestyle changes and in some cases, surgery. Adequately controlled, Crohn's disease may not significantly restrict daily living.[77] Treatment for Crohn's disease is only when symptoms are active and involve first treating the acute problem, then maintaining remission.
Medication
Acute treatment uses medications to treat any infection (normally antibiotics) and to reduce inflammation (normally aminosalicylate anti-inflammatory drugs and corticosteroids). When symptoms are in remission, treatment enters maintenance with a goal of avoiding the recurrence of symptoms. Prolonged use of corticosteroids has significant side-effects; as a result they are generally not used for long-term treatment. Alternatives include aminosalicylates alone, though only a minority are able to maintain the treatment, and many require immunosuppressive drugs.[74]
Medications used to treat the symptoms of Crohn's disease include 5-aminosalicylic acid (5-ASA) formulations, prednisone, immunomodulators such as azathioprine, mercaptopurine, methotrexate, infliximab, adalimumab[14] and natalizumab.[78][79] Hydrocortisone should be used in severe attacks of Crohn's disease.[80]
Lifestyle changes
Certain lifestyle changes can reduce symptoms, including dietary adjustments, proper hydration and smoking cessation.[77]
Surgery
Crohn's cannot be cured by surgery, though it is used when partial or a full blockage of the intestine occurs. Surgery may also be required for complications such as obstructions, fistulas and/or abscesses, or if the disease does not respond to drugs within a reasonable time. After the first surgery, Crohn's usually shows up at the site of the resection though it can appear in other locations. After a resection, scar tissue builds up which causes strictures. A stricture is when the intestines becomes too small to allow excrement to pass through easily which can lead to a blockage. After the first resection, another resection may be necessary within five years.[81] For patients with an obstruction due to a stricture, two options for treatment are strictureplasty and resection of that portion of bowel. There is no statistical significance between strictureplasty alone versus strictureplasty and resection in cases of duodenal involvement. In these cases, re-operation rates were 31% and 27%, respectively, indicating that strictureplasty is a safe and effective treatment for selected patients with duodenal involvement.[82]
Short bowel syndrome (SBS, also short gut syndrome or simply short gut) can be caused by the surgical removal of the small intestines. It usually develops if a person has had half or more of their small intestines removed.[83] Diarrhea is the main symptom of short bowel syndrome though other symptoms may include cramping, bloating and heartburn. Short bowel syndrome is treated with changes in diet, intravenous feeding, vitamin and mineral supplements and treatment with medications.
Prospective treatments
Researchers at University College London have questioned the wisdom of suppressing the immune system in Crohn's, as the problem may be an under-active rather than an over-active immune system: their study found that Crohn's patients showed an abnormally low response to an introduced infection, marked by a poor flow of blood to the wound, and the response improved when the patients were given sildenafil.[84]
Recent studies using helminthic therapy or hookworms to treat Crohn's Disease and other (non-viral) auto-immune diseases seem to yield promising results.[85]
A single, small, uncontrolled trial of patients with mild Crohn's on stable medications suggested improvement with low dose naltrexone therapy.[86]
A recent small double-blind, placebo-controlled study[87] conducted in Germany found grand wormwood to have a steroid-sparing effect with the result that 18/20 wormwood receiving patients were able to taper steroid medication while maintaining a steady improvement in CD symptoms.
Recent research in France has suggested that a shortage of the bacterium Faecalibacterium prausnitzii may cause Crohn's disease by overstimulating the immune system. The researchers said that if ongoing animal trials prove successful, human patients could benefit from a probiotic treatment with F. prausnitzii.[88]
Prognosis
Crohn's disease is a chronic condition for which there is currently no cure. It is characterised by periods of improvement followed by episodes when symptoms flare up. With treatment, most people achieve a healthy height and weight, and the mortality rate for the disease is low. Crohn's disease is associated with an increased risk of small bowel and colorectal carcinoma.[89]
Epidemiology
The incidence of Crohn's disease has been ascertained from population studies in Norway and the United States and is similar at 6 to 7.1:100,000.[90][91] Crohn's disease is more common in northern countries, and shows a higher preponderance in northern areas of the same country.[92] The incidence of Crohn's disease is thought to be similar in Europe but lower in Asia and Africa.[90] It also has a higher incidence in Ashkenazi Jews.[14]
Crohn's disease has a bimodal distribution in incidence as a function of age: the disease tends to strike people in their teens and 20s, and people in their 50s through to their 70s, and ages in between due to not being diagnosed with Crohn's and being diagnosed instead with irritable bowel syndrome (IBS).[1][7] It is rarely diagnosed in early childhood. It usually strikes females who are pediatric patients more severely than males.[93] However, only slightly more women than men have Crohn's disease.[94] Parents, siblings or children of people with Crohn's disease are 3 to 20 times more likely to develop the disease.[95] Twin studies show a concordance of greater than 55% for Crohn's disease.[96]
History
Inflammatory bowel diseases were described by Giovanni Battista Morgagni (1682-1771), by Polish surgeon Antoni Leśniowski in 1904 (leading to the use of the eponym "Leśniowski-Crohn disease" in Poland) and by Scottish physician T. Kennedy Dalziel in 1913.[97]
Burrill Bernard Crohn, an American gastroenterologist at New York City's Mount Sinai Hospital, described fourteen cases in 1932, and submitted them to the American Medical Association under the rubric of "Terminal ileitis: A new clinical entity". Later that year, he, along with colleagues Leon Ginzburg and Gordon Oppenheimer published the case series as "Regional ileitis: a pathologic and clinical entity".[9]
See also
- The Crohn's and Colitis Foundation of Canada
- Crohn's and Colitis Foundation of America
- Johne's disease
- Mycobacterium avium subspecies paratuberculosis
- Ulcerative colitis
- Colostomy
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 Hanauer, Stephen B. (1996). "Inflammatory bowel disease". New England Journal of Medicine 334 (13): 841–8. doi:. PMID 8596552.
- ↑ Mayo Clinic: Crohn's Disease
- ↑ National Digestive Diseases Information Clearinghouse
- ↑ Nicotine and IBD - Smoking and IBD
- ↑ Loftus, E. V.; P. Schoenfeld, W. J. Sandborn (2002). "The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review". Alimentary Pharmacology & Therapeutics 16 (1): 51–60. doi:. PMID 11856078.
- ↑ 6.0 6.1 Bernstein, Charles N. (2006). The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. 101. pp. 1559–68. doi:. PMID 16863561.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Gopal, Latha; Senthil Nachimuthu (2006-05-23). "Crohn Disease". eMedicine. Retrieved on 2006-07-02.
- ↑ Al-Ataie, M Bashar; Vishwanath N Shenoy (2005-10-04). "Ulcerative colitis". eMedicine. Retrieved on 2006-07-02.
- ↑ 9.0 9.1 9.2 Crohn BB, Ginzburg L, Oppenheimer GD (2000). "Regional ileitis: a pathologic and clinical entity. 1932". Mt. Sinai J. Med. 67 (3): 263–8. PMID 10828911.
- ↑ 10.0 10.1 Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer S, Irvine E, Jewell D, Rachmilewitz D, Sachar D, Sandborn W, Sutherland L (2000). "A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998". Inflamm Bowel Dis 6 (1): 8–15. PMID 10701144.
- ↑ Dubinsky MC, Fleshner PP. (2003). "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Curr Treat Options Gastroenterol 6 (3): 183–200. doi:. PMID 12744819.
- ↑ 12.0 12.1 Pimentel, Mark; Michael Chang, Evelyn J. Chow, Siamak Tabibzadeh, Viorelia Kirit-Kiriak, Stephan R. Targan, Henry C. Lin (2000). "Identification of a prodromal period in Crohn's disease but not ulcerative colitis". American Journal of Gastroenterology 95 (12): 3458–62. doi:. PMID 11151877.
- ↑ Crohn's Disease Overview
- ↑ 14.0 14.1 14.2 14.3 Podolsky, Daniel K. (2002). "Inflammatory bowel disease". New England Journal of Medicine 346 (6): 417–29. doi:. PMID 12167685. http://content.nejm.org/cgi/content/extract/347/6/417. Retrieved on 2006-07-02.
- ↑ Mueller, M. H.; M. E. Kreis, M. L. Gross, H. D. Becker, T. T. Zittel & E. C. Jehle (2002). "Anorectal functional disorders in the absence of anorectal inflammation in patients with Crohn's disease". British Journal of Surgery 89 (8): 1027–31. doi:. PMID 12153630.
- ↑ Taylor B, Williams G, Hughes L, Rhodes J (1989). "The histology of anal skin tags in Crohn's disease: an aid to confirmation of the diagnosis". Int J Colorectal Dis 4 (3): 197–9. doi:. PMID 2769004.
- ↑ Fix, Oren K.; Jorge A. Soto, Charles W. Andrews and Francis A. Farraye (2004). "Gastroduodenal Crohn's disease". Gastrointestinel Endoscopy 60 (6): 985. doi:. PMID 15605018.
- ↑ 18.0 18.1 Beattie, R.M.; N. M. Croft, J. M. Fell, N. A. Afzal and R. B. Heuschkel (2006). "Inflammatory bowel disease". Archives of Disease in Childhood 91 (5): 426–32. doi:. PMID 16632672.
- ↑ Büller, H.A. (1997). "Problems in diagnosis of IBD in children". The Netherlands Journal of Medicine 50 (2): S8–S11. doi:. PMID 9050326.
- ↑ O'Keefe, S. J. (1996). "Nutrition and gastrointestinal disease". Scandinavian Journal of Gastroenterology Supplement 31 (220): 52–9. doi:. PMID 8898436.
- ↑ Danese, Silvio; Stefano Semeraro, Alfredo Papa, Italia Roberto, Franco Scaldaferri, Giuseppe Fedeli, Giovanni Gasbarrini, Antonio Gasbarrini (2005). "Extraintestinal manifestations in inflammatory bowel disease". World Journal of Gastroenterology 11 (46): 7227–36. PMID 16437620. http://www.wjgnet.com/1007-9327/11/7227.asp. Retrieved on 2006-07-02.
- ↑ 22.0 22.1 Crohn's disease. professionals.epilepsy.com. Retrieved on July 13, 2007.
- ↑ MedlinePlus Encyclopedia Small bowel bacterial overgrowth
- ↑ Ekbom A, Helmick C, Zack M, Adami H (1990). "Increased risk of large-bowel cancer in Crohn's disease with colonic involvement". Lancet 336 (8711): 357–9. doi:. PMID 1975343.
- ↑ Collins P, Mpofu C, Watson A, Rhodes J (2006). "Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease". Cochrane Database Syst Rev: CD000279. doi:. PMID 16625534.
- ↑ Lynne V McFarland (2008). "Colorectal cancer and dysplasia in inflammatory bowel disease". World Journal of Gastroenterology: 2665.
- ↑ Evans J, Steinhart A, Cohen Z, McLeod R (2003). "Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease". J Gastrointest Surg 7 (4): 562–6. doi:. PMID 12763417.
- ↑ "Complications of Crohn's Disease". Retrieved on 2008-01-16.
- ↑ Kaplan, C (2005-10-21). "IBD and Pregnancy: What You Need to Know". Crohn's and Colitis Foundation of America. Retrieved on 2008-02-14.
- ↑ "Crohn's disease has strong genetic link: study". Crohn's and Colitis Foundation of America (2007-04-16). Retrieved on 2008-04-20.
- ↑ 31.0 31.1 31.2 "Possible links between Crohn’s disease and Paratuberculosis" (PDF). EUROPEAN COMMISSION DIRECTORATE-GENERAL HEALTH & CONSUMER PROTECTION.
- ↑ Ogura Y, Bonen DK, Inohara N, et al (2001). "A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease". Nature 411 (6837): 603–6. doi:. PMID 11385577.
- ↑ Cuthbert A, Fisher S, Mirza M, et al. (2002). "The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease". Gastroenterology 122 (4): 867–74. doi:. PMID 11910337.
- ↑ Kaser et. al. (5 September 2008). "XBP1 Links ER Stress to Intestinal Inflammation and Confers Genetic Risk for Human Inflammatory Bowel Disease". Cell (Cell Press) 134: 743-756. http://www.cell.com/content/article/abstract?uid=PIIS0092867408009410.
- ↑ Cenac N, Andrews CN, Holzhausen M, et al (March 2007). "Role for protease activity in visceral pain in irritable bowel syndrome". J. Clin. Invest. 117 (3): 636–47. doi:. PMID 17304351.
- ↑ Cenac N, Coelho AM, Nguyen C, et al (November 2002). "Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2". Am. J. Pathol. 161 (5): 1903–15. PMID 12414536. http://ajp.amjpathol.org/cgi/pmidlookup?view=long&pmid=12414536.
- ↑ Boorom KF, Smith H, Nimri L, et al (October 2008). "Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection". Parasit Vectors 1 (1): 40. doi:. PMID 18937874.
- ↑ Sakamoto N, Kono S, Wakai K, et al. (2005). "Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan". Inflamm Bowel Dis 11 (2): 154–63. doi:. PMID 15677909.
- ↑ Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T (1997). "Pre-illness dietary factors in inflammatory bowel disease" (PDF). Gut 40 (6): 754–60. doi:10.1136/gut.40.6.754 (inactive 2008-06-25). PMID 9245929. http://gut.bmj.com/cgi/reprint/40/6/754.
- ↑ Cosnes J (2004). "Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice". Best Pract Res Clin Gastroenterol 18 (3): 481–96. doi:. PMID 15157822.
- ↑ Lesko S, Kaufman D, Rosenberg L, et al. (1985). "Evidence for an increased risk of Crohn's disease in oral contraceptive users". Gastroenterology 89 (5): 1046–9. PMID 4043662.
- ↑ Morris, Danielle L; Scott M Montgomery (2000-11-18). "Early environmental factors may have role in both Crohn's disease and gastric carcinoma - Letter to the Editor". British Medical Journal. http://findarticles.com/p/articles/mi_m0999/is_7271_321/ai_67708495/. Retrieved on 2008-01-16.
- ↑ Cobrin GM, Abreu MT (2005). "Defects in mucosal immunity leading to Crohn's disease". Immunol. Rev. 206: 277–95. doi:. PMID 16048555.
- ↑ 44.0 44.1 Elson, C. (2007), "Monoclonal Anti–Interleukin 23 Reverses Active Colitis in a T Cell–Mediated Model in Mice", Gastroenterology 132: 2359, doi:
- ↑ Prescott NJ, Fisher SA, Franke A, et al (2007). "A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5". Gastroenterology 132 (5): 1665–71. doi:. PMID 17484864.
- ↑ "Crohns Research Overview Page".
- ↑ Sartor, R. (2006). "Mechanisms of Disease: pathogenesis of Crohn's disease and ulcerative colitis". Nature Clinical Practice Gastroenterology & Hepatology (3): 390–407. doi:10.1038 (inactive 2008-06-25).
- ↑ Naser SA, Collins MT (2005). "Debate on the lack of evidence of Mycobacterium avium subsp. paratuberculosis in Crohn's disease". Inflamm. Bowel Dis. 11 (12): 1123. PMID 16306778.
- ↑ Giaffer MH, Clark A, Holdsworth CD (1992). "Antibodies to Saccharomyces cerevisiae in patients with Crohn's disease and their possible pathogenic importance". Gut 33 (8): 1071–5. doi:. PMID 1398231.
- ↑ "Paratuberculosis And Crohn's Disease: Got Milk?".
- ↑ "Two-year outcomes analysis of Crohn's Disease treated with rifabutin and macrolide antibiotics.".
- ↑ Baumgart, M., et al. (2007). "Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum (advance online publication)". The ISME Journal 1: 403. doi:. http://search.nature.com/search/?sp-a=sp1001702d&sp-sfvl-field=subject. – Scholar search
- ↑ "Scientists find how bacteria in cows milk may cause Crohn's disease". University of Liverpool.
- ↑ "Washington Post: Roche Found Liable". Retrieved on 2008-06-21.
- ↑ Reddy D, Siegel CA, Sands BE, Kane S (2006). "Possible association between isotretinoin and inflammatory bowel disease". Am. J. Gastroenterol. 101 (7): 1569–73. doi:. PMID 16863562.
- ↑ Borobio E, Arín A, Valcayo A, Iñarrairaegui M, Nantes O, Prieto C (2004). "[Isotretinoin and ulcerous colitis]" (in Spanish; Castilian). An Sist Sanit Navar 27 (2): 241–3. PMID 15381956.
- ↑ Reniers DE, Howard JM (2001). "Isotretinoin-induced inflammatory bowel disease in an adolescent". Ann Pharmacother 35 (10): 1214–6. PMID 11675849. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11675849.
- ↑ "Jury Awards $10.5 Million Over Accutane". Retrieved on 2008-06-21.
- ↑ Crawford JM. "The Gastrointestinal tract, Chapter 17". In Cotran RS, Kumar V, Robbins SL. Robbins Pathologic Basis of Disease: 5th Edition. W.B. Saunders and Company, Philadelphia, 1994.
- ↑ HCP: Pill Cam, Capsule Endoscopy, Esophageal Endoscopy
- ↑ 61.0 61.1 Hara, Amy K.; Jonathan A. Leighton, Russell I. Heigh, Virender K. Sharma, Alvin C. Silva, Giovanni De Petris, Joseph G. Hentz and David E. Fleischer (2006). "Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy". Radiology 238 (1): 128–34. doi:. PMID 16373764.
- ↑ Triester, Stuart L.; Jonathan A. Leighton, Grigoris I. Leontiadis, Suryakanth R. Gurudu, David E. Fleische, Amy K. Hara, Russell I. Heigh, Arthur D. Shiff, and Virender K. Sharma (2006). "A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. This cannot and should not be used if a stricture or blockage is known, pictures should be taken prior to using the camera since it can get stuck and surgery in the only way to remove it". The American Journal of Gastroenterology 101 (5): 954–64. doi:. PMID 16696781.
- ↑ Dixon, P.M.; M.E. Roulston and D.J. Nolan (1993). "The small bowel enema: a ten year review". Clinical Radiology 47 (1): 46–8. doi:. PMID 8428417.
- ↑ Carucci, L. R.; M. S. Levine (2002). "Radiographic imaging of inflammatory bowel disease". Gastroenterology Clinics of North America 31 (1): 93–117. doi:. PMID 12122746.
- ↑ Rajesh, A.; D.D.T. Maglinte (2006). "Multislice CT enteroclysis: technique and clinical applications". Clinical Radiology 61 (1): 31–9. doi:. PMID 16356814.
- ↑ Zissin, Rivka; Marjorie Hertz, Alexandra Osadchy, Ben Novis and Gabriela Gayer (2005). "Computed Tomographic Findings of Abdominal Complications of Crohn’s Disease—Pictorial Essay" (PDF). Canadian Association of Radiologists Journal 56 (1): 25–35. PMID 15835588. http://www.carj.ca/issues/2005-Feb/25/pg25.pdf. Retrieved on 2006-07-02.
- ↑ MacKalski, B. A.; C. N. Bernstein (2005). "New diagnostic imaging tools for inflammatory bowel disease". Gut 55 (5): 733–41. doi:. PMID 16609136.
- ↑ Goh, Jason; C. A. O'Morain (2003). "Review article: nutrition and adult inflammatory bowel disease". Alimentary Pharmacology & Therapeutics 17 (3): 307–20. doi:. PMID 12562443.
- ↑ Chamouard, Patrick; Zoe Richert, Nicolas Meyer, Gabriel Rahmi, René Baumann (2006). "Diagnostic Value of C-Reactive Protein for Predicting Activity Level of Crohn's Disease". Clinical Gastroenterology and Hepatology 4: 882. doi:. PMID 16630759. Epub ahead of print
- ↑ Kaila, B.; K. Orr and C. N. Bernstein (2005). "The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn's disease and predicting inflammatory disease". The Canadian Journal of Gastroenterology 19 (12): 717–21. PMID 16341311. http://www.pulsus.com/Gastro/19_12/kail_ed.htm. Retrieved on 2006-07-02.
- ↑ Israeli, E.; I. Grotto, B. Gilburd, R. D. Balicer, E. Goldin, A. Wiik and Y. Shoenfeld (2005). "Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease". Gut 54 (9): 1232–6. doi:. PMID 16099791.
- ↑ Faecal calprotectin: a bright future for assessing disease activity in Crohn's disease. Gaya et al. Q J Med 2002; 95: 557-558
- ↑ 73.0 73.1 73.2 73.3 Kornbluth, Asher; David B. Sachar (2004). "Ulcerative Colitis Practice Guidelines in Adults" (PDF). American Journal of Gastroenterology 99 (7): 1371–85. doi:. PMID 15233681. http://www.acg.gi.org/physicians/guidelines/UlcerativeColitisUpdate.pdf. Retrieved on 2006-11-08.
- ↑ 74.0 74.1 74.2 74.3 74.4 Hanauer, Stephen B.; William Sandborn (2001-03-01). "Management of Crohn's Disease in Adults" (PDF). American Journal of Gastroenterology 96 (3): 635–43. doi:. PMID 11280528. http://www.acg.gi.org/physicians/guidelines/CrohnsDiseaseinAdults.pdf. Retrieved on 2006-11-08.
- ↑ Broomé, Ulrika; Annika Bergquist (2006). "Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer". Seminars in Liver Disease 26 (1): 31–41. doi:. PMID 16496231.
- ↑ "Clinical Research Alliance Update" (pdf). Crohn's and Colitis Foundation of America (2007-05-01). Retrieved on 2008-02-14.
- ↑ 77.0 77.1 Fries, WS (2007-05-16). "Crohn's Disease: 54 Tips to Help You Manage". WebMD. Retrieved on 2008-02-14.
- ↑ Sandborn, W.J.; Colombel, J.F.; Enns, R.; Feagan, B.G.; Hanauer, S.B.; Lawrance, I.C.; Panaccione, R.; Sanders, M.; Schreiber, S.; Targan, S.; Others, (2005). "Natalizumab Induction and Maintenance Therapy for Crohn's Disease". New England Journal of Medicine 353 (18): 1912. doi:. PMID 16267322.
- ↑ MacDonald, J.K.; McDonald, J.W.D. (2006). "Natalizumab for induction of remission in Crohn's disease (Cochrane Review)". The Cochrane Database of Systematic Reviews: 1465–858. doi:. http://dellboy.update-software.com/abstracts/AB006097.htm. Retrieved on 2008-02-15.
- ↑ Longmore, Murray; Ian Wilkinson, Tom Turmezei, Chee Kay Cheung (2007). Oxford Handbook of Clinicial Medicine, 7th edition. Oxford University Press. pp. 266–7. ISBN 0-19-856837-1.
- ↑ Tresca, AJ (2007-01-12). "Resection Surgery for Crohn's Disease". About.com. Retrieved on 2008-02-14.
- ↑ Ozuner G, Fazio VW, Lavery IC, Milsom JW, Strong SA (1996). "Reoperative rates for Crohn's disease following strictureplasty. Long-term analysis". Dis. Colon Rectum 39 (11): 1199–203. doi:. PMID 8918424.
- ↑ Short Bowel Syndrome as defined by the National Institute of Diabetes and Digestive and Kidney Diseases
- ↑ Segal AW et al (2006). "Defective acute inflammation in Crohn's disease: a clinical investigation". Lancet 367 (9511): 668–78. doi:.
- ↑ Croese J, O'neil J, Masson J, et al (2006). "A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors". Gut 55 (1): 136–7. doi:. PMID 16344586.
- ↑ Smith J, Stock H, Bingaman S et al (2007). "Low-Dose Naltrexone Therapy Improves Active Crohn’s Disease". American Journal of Gastroenterology 102 (4): 820–8. doi:. PMID 17222320.
- ↑ B. Omera, S. Krebsb, H. Omerc and T.O. Noord (2007). "Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn's disease: A double-blind placebo-controlled study". Phytomedicine 14 (2-3): 87–95. doi:.
- ↑ "Bacterium 'to blame for Crohn's'". news.bbc.co.uk. Retrieved on Nov. 23,2008.
- ↑ 90.0 90.1 Hiatt, Robert A.; Leon Kaufman (1988). "Epidemiology of inflammatory bowel disease in a defined northern California population". Western Journal of Medicine 149 (5): 541–6. PMID 3250100.
- ↑ Moum, B.; M. H. Vatn, A. Ekbom, E. Aadland, O. Fausa, I. Lygren, N. Stray, J. Sauar, T. Schulz (1996). "Incidence of Crohn's disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists". Scandinavian Journal of Gastroenterology 31 (4): 355–61. doi:. PMID 8726303.
- ↑ Shivananda, S.; J. Lennard-Jones, R. Logan, N. Fear, A. Price, L. Carpenter and M. van Blankenstein (1996). "Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD)". Gut 39 (5): 690–7. doi:. PMID 9014768.
- ↑ "Crohn's disease manifests differently in boys and girls". CCFA.org.
- ↑ "Who is affected by Crohn's disease". WebMD.com.
- ↑ Satsangi J, Jewell DP, Bell JI (1997). "The genetics of inflammatory bowel disease". Gut 40 (5): 572–4. PMID 9203931. PMC: 1027155. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=9203931.
- ↑ Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B (1988). "Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking". Gut 29 (7): 990–6. PMID 3396969. PMC: 1433769. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=3396969.
- ↑ Kirsner JB (1988). "Historical aspects of inflammatory bowel disease". J. Clin. Gastroenterol. 10 (3): 286–97. PMID 2980764.
External links
- Crohn's disease at the Open Directory Project
|
|||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
