Clostridium tetani
| Clostridium tetani | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
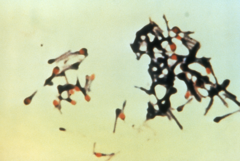 Clostridium tetani with characteristic 'tennis racket' appearance.
|
||||||||||||||
| Scientific classification | ||||||||||||||
|
||||||||||||||
| Binomial name | ||||||||||||||
| Clostridium tetani Flügge, 1886 |
Clostridium tetani is a rod-shaped, anaerobic bacterium of the genus Clostridium. Like other Clostridium species, it is Gram-positive, and its appearance on a gram stain resembles tennis rackets or drumsticks.[1] C. tetani is found as spores in soil or as parasites in the gastrointestinal tract of animals. C. tetani produces a potent biological toxin, tetanospasmin, and is the causative agent of tetanus.
Contents |
History
Tetanus was well known to ancient peoples, who recognized the relationship between wounds and fatal muscle spasms. In 1884, Arthur Nicolaier isolated the strychnine-like toxin of tetanus from free-living, anaerobic soil bacteria. The etiology of the disease was further elucidated in 1884 by Antonio Carle and Giorgio Rattone, who demonstrated the transmissibility of tetanus for the first time. They produced tetanus in rabbits by injecting their sciatic nerve with pus from a fatal human tetanus case in that same year. In 1889, C. tetani was isolated from a human victim, by Kitasato Shibasaburo, who later showed that the organism could produce disease when injected into animals, and that the toxin could be neutralized by specific antibodies. In 1897, Edmond Nocard showed that tetanus antitoxin induced passive immunity in humans, and could be used for prophylaxis and treatment. Tetanus toxoid vaccine was developed by P. Descombey in 1924, and was widely used to prevent tetanus induced by battle wounds during World War II.[2]
Characteristics
C. tetani is a rod-shaped, obligate anaerobe which stains Gram positive in fresh cultures; established cultures may stain Gram negative.[1] During vegetative growth, the organism cannot survive in the presence of oxygen, is sensitive to heat and has flagella which provide limited mobility. As the bacterium matures, it develops a terminal spore, which gives the organism its characteristic appearance. C. tetani spores are extremely hardy, and are resistant to heat and most antiseptics.[3] The spores are distributed widely in manure-treated soils, and can also be found on human skin and in contaminated heroin .[2]
Toxicity
C. tetani usually enters a host through a wound to the skin and then it replicates. Once an infection is established, C. tetani produces two exotoxins, tetanolysin and tetanospasmin. Eleven strains of C. tetani have been identified, which differ primarily in flagellar antigens and in its ability to produce tetanospasmin. The genes that produce toxin are encoded on a plasmid which is present in all toxigenic strains, and all strains that are capable of producing toxin produce identical toxins.[4]
Tetanolysin serves no known function to C. tetani, and the reason the bacteria produce it is not known with certainty. Tetanospasmin is a neurotoxin and causes the clinical manifestations of tetanus. Tetanus toxin is generated in living bacteria, and is released when the bacteria lyses, such as during spore germination or during vegetative growth. A minimal amount of spore germination and vegetative cell growth are required for toxin production.[4]
On the basis of weight, tetanospasmin is one of the most potent toxins known. The estimated minimum human lethal dose is 2.5 nanograms per kilogram of body weight, or 175 nanograms in a 70 kg (154 lb) human.[2] The only toxins more lethal to humans are botulinum toxin, produced by close relative Clostridium botulinum and the exotoxin produced by Corynebacterium diphtheriae, the causative agent of diphtheria.
Tetanospasmin is a zinc-dependent metalloproteinase, that is similar in structure to botulinum toxin, but each toxin produces quite different effects. C. tetani synthesizes tetanospasmin as a single 150kDa polypeptide progenitor toxin, that is then cleaved by a protease into two fragments; fragment A (a 50kDa "light chain") and fragment B (a 100 kDa heavy chain) which remain connected via a disulfide bridge. Cleavage of the progenitor toxin into A and B fragments can also be induced artificially with trypsin.[4]
Toxin Action
Tetanospasmin is distributed in the blood and lymphatic system of the host. The toxin acts at several sites within the central nervous system, including peripheral nerve terminals, the spinal cord, and brain, and within the sympathetic nervous system. The toxin is taken up into within the nerve axon and transported across synaptic junctions, until it reaches the central nervous system, where it is rapidly fixed to gangliosides at the presynaptic junctions of inhibitory motor nerve endings.[2]
The clinical manifestations of tetanus are caused when tetanus toxin blocks inhibitory impulses, by interfering with the release of neurotransmitters, including glycine and gamma-aminobutyric acid. This leads to unopposed muscle contraction and spasm. Characteristic features are Risus Sardonicus (a rigid smile), Trismus (commonly known as lock-jaw), and Opisthotonus (rigid, arched back). Seizures may occur, and the autonomic nervous system may also be affected. Tetanospasmin appears to prevent the release of neurotransmitters by selectively cleaving a component of synaptic vesicles called synaptobrevin II.[4]
It should be noted that the organism itself has no access to the nervous system, and yet tetanospasmin is directed toward the nervous system. The reason why this occurs, is still a subject of controversy. It's fairly known that toxins are by-products synthesized during bacterial growth, and their targets are determined by the presence or absence of specific receptors on human cells to which they can bind and exert their effect. This only explains why the tetanus toxin acts on the nervous system, but why it reaches a place to which the organism itself has no access may be an anomaly of nature.
Treatment
When a tetanus infection becomes established, treatment usually focuses on controlling muscle spasms, stopping toxin production, and neutralizing the effects of the toxin. Treatment includes administration of tetanus immune globulin (TIG), which comprises antibodies that inhibit tetanus toxin (also known as tetanus antitoxins), by binding to and removing unbound tetanus toxin from the body. Binding of the toxin to the nerve endings appears to be an irreversible event, and TIG is ineffective at removing bound toxin. Recovery of affected nerves requires the sprouting of a new axon terminal.[4] Large doses of antibiotic drugs (such as metronidazole or intramuscular penicillin G) are also given once tetanus infection is suspected, to halt toxin production.
Prevention of tetanus includes vaccination, and cleaning the primary wound. Prophylaxis is effective, in the form of a tetanus toxoid vaccine, which is given with or without passive immunization with tetanus immune globulin. Very few cases of tetanus have occurred in individuals with up-to-date tetanus vaccinations. DPT vaccine (diphtheria-pertussis-tetanus) in North America, is given at 2, 4, 6, and 15–18 months of age, followed by a booster before entry to school (4-6 years). This regimen provides protection from tetanus for about 10 years, and every 10 years thereafter, a booster shot of tetanus vaccine is recommended.[2]
Tetanus is not contagious from person to person, and is the only vaccine-preventable disease that is infectious but not contagious. A C. tetani infection does not result in tetanus immunity, and tetanus vaccination should be given as soon as the patient has stabilized.[2]
References
- ↑ 1.0 1.1 Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. ISBN 0838585299.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Atkinson W, Hamborsky J, McIntyre L, Wolfe S (eds). (2006). Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (9th ed. ed.). Public Health Foundation. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/tetanus.pdf.
- ↑ Madigan M; Martinko J (editors). (2005). Brock Biology of Microorganisms (11th ed. ed.). Prentice Hall. ISBN 0131443291.
- ↑ 4.0 4.1 4.2 4.3 4.4 Todar, Ken (2005) Pathogenic Clostridia Ken Todar's Microbial World. University of Wisconsin - Madison.
Further reading
- Clinical Microbiology, ISBN 0-940780-49-6