Ciprofloxacin
 |
|
 |
|
|
Ciprofloxacin
|
|
| Systematic (IUPAC) name | |
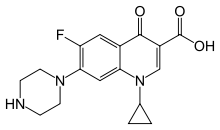
| 1-cyclopropyl-6-fluoro-4-oxo- 7-piperazin-1-yl-quinoline-3-carboxylic acid |
|
| Identifiers | |
| CAS number | |
| ATC code | J01 S01 S03 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C17H18FN3O3 |
| Mol. mass | 331.346 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 69%[1] |
| Metabolism | Hepatic, including CYP1A2 |
| Half life | 4 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral, intravenous, topical (ear drops, eye drops) |
Ciprofloxacin (INN) is a synthetic antibiotic, originally manufactured and sold by Bayer A.G.. Generic versions are now available, but Bayer continues sale under the brand names Cipro, Ciproxin and Ciprobay (and other brand names in other markets, e.g. Veterinary medicine), belonging to a group called fluoroquinolones. Ciprofloxacin is bactericidal. Its mode of action depends upon blocking bacterial DNA replication by binding itself to an enzyme called DNA gyrase, thereby inhibiting the unwinding of bacterial chromosomal DNA during and after the replication.
Ciprofloxacin is available for oral, parenteral and topical use. It has a variety of indications, including lower respiratory tract infections (such as pneumonia and acute bronchitis), urinary tract infections, several STDs, skin and soft tissue infections, septicemia, legionellosis, and anthrax.
Contents |
Activity
Ciprofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase iv[2], which is an enzyme necessary to separate replicated DNA, thereby inhibiting cell division. It is effective against:
- Enterobacteriaceae
- Vibrio
- Haemophilus influenzae
- Haemophilus ducreyi
- Neisseria gonorrhoeae (widespread resistance to ciprofloxacin limits its usefulness in treating N. gonorrhea infections)
- Neisseria meningitidis
- Moraxella catarrhalis
- Brucella
- Campylobacter
- Mycobacterium intracellulare
- Legionella sp.
- Pseudomonas aeruginosa
- Bacillus anthracis
- Escherichia coli
- Staphylococcus aureus (MRSA)[3]
Weak activity against:
- Streptococcus pneumoniae
- Chlamydia trachomatis
- Chlamydia pneumoniae
No activity against:
- Bacteroides
- Burkholderia cepacia
- Enterococcus faecium
- Ureaplasma urealyticum
- Streptococcus pyogenes
- and others
Use against chlamydia and mycoplasma infections is now contraindicated; ciprofloxacin appears to be ineffective against these organisms, merely stopping their growth (and allowing them to resume growth after the antibiotic is withdrawn) rather than killing them. [4]
Contraindications
Medications containing metals, such as aluminium, magnesium, calcium, ferrous sulfate, iron, and zinc, are thought to form chelation complexes with fluoroquinolone antibiotics and prevent the drugs from being absorbed. Because of this, avoid taking ciprofloxacin with antacids which contain aluminium, magnesium or calcium. Sucralfate, which has a high aluminium content, also reduces the bioavailability of ciprofloxacin to approximately 4%.[5] Ciprofloxacin may be taken with meals or on an empty stomach. Ciprofloxacin should not be taken with dairy products or calcium-fortified juices alone, but may be taken with a meal that contains these products.[6]
Heavy exercise is discouraged, as achilles tendon rupture has been reported in patients taking ciprofloxacin. Achilles tendon rupture due to ciprofloxacin use is typically associated with renal failure.
Fluoroquinolones are increasingly contraindicated for patients who have been to S.E. Asia due to the growing prevalence of antibiotic resistance to the class of antibiotics in that region.[7]
Ciprofloxacin is also contraindicated in children (except for serious infections and anthrax post-exposure), pregnancy, and in patients with epilepsy. Dose adjustment or avoidance may be necessary with liver or renal failure.
Adverse effects
Manufacturer-funded studies report that approximately 9% of patients taking the medication experience side effects ranging from mild to moderate, with the vast majority of those relating to metabolic-nutritional problems and the central nervous system.[8] Compared with other fluoroquinolones the incidence and severity of side effects from ciprofloxacin is low;[9] the major adverse effect most often seen with its use is gastrointestinal irritation, as is common with many antibiotics. Because of its general safety, potency and broad spectrum of activity, ciprofloxacin was initially reserved as a drug of last resort for use against difficult-to-treat and antibiotic-resistant infections. As with any antibiotic, however, increasing time and use has led to an increase in ciprofloxacin-resistant infections, mainly in the hospital setting. Also implicated in the rise of resistant bacteria is the use of lower-cost, less potent fluoroquinolones, and the widespread addition of ciprofloxacin and other antibiotics to the feed of farm animals, which leads to greater and more rapid weight gain for unclear reasons.
Ciprofloxacin can cause photosensitivity reactions and can elevate plasma theophylline levels to toxic values. It can also cause constipation and sensitivity to caffeine. Ciprofloxacin is also known to cause swelling of joints and cartilage, and cause tendon rupture and chronic pain.
In 2005 the U.S. Food and Drug Administration (FDA) changed the package insert for Cipro[10] to acknowledge the tendon ruptures and the development of irreversible neurological conditions. On 8 July 2008, as the result of a lawsuit filed by Public Citizen, the FDA upgraded the warning to a "black box" for fluoroquinolones, including ciprofloxacin.[11]
The incidence of side effects for ciprofloxacin is widely believed by physicians to be acceptable and relatively safe. However, the safety of Cipro has been increasingly challenged with a large number of patients suffering serious adverse effects with disabling effects lasting months or years with higher doses and/or longer duration of administration. [12]
See also quinolone#Adverse effects.
Interactions
The toxicity of drugs that are metabolised by the cytochrome P450 system is enhanced by concomitant use of some quinolones. Coadministration may dangerously increase coumadin warfarin activity; INR should be monitored closely. They may also interact with the GABA A receptor and cause neurological symptoms; this effect is augmented by certain non-steroidal anti-inflammatory drugs.[13] Quercetin, a flavonoid occasionally used as a dietary supplement, may interact with fluoroquinolones, as quercetin competitively binds to bacterial DNA gyrase. Some foods such as garlic and apples contain high levels of quercetin; whether this inhibits or enhances the effect of fluoroquinolones is not entirely clear.[14]
Dosing
Ciprofloxacin is available in oral tablets (250, 500, 750, and 1000 mg), as well as ready-made infusion bottles (200 and 400 mg). A combination preparation of ciprofloxacin 500 mg and tinidazole 600 mg is marketed under the name Ciplox-TZ for infections where anaerobes or protozoa together with ciprofloxacin-sensitive aerobes are likely. Dosage in respiratory tract infections is 500–1500 mg a day in 2 doses.
Due to its elimination half-life, ciprofloxacin is administered twice daily. No dose adjustments are generally required for mild to moderate renal impairment.

References
- ↑ Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J. Absolute oral bioavailability of ciprofloxacin. Antimicrob Agents Chemother 1986;30:444-6. PMID 3777908.
- ↑ Drlica, K., and X. Zhao. 1997. DNA Gyrase, Topoisomerase IV, and the 4-Quinolones. Microbiology and Molecular Biology Reviews. Sept. p. 377–392.
- ↑ Venezio FR, Tatarowicz W, DiVincenzo CA, and O'Keefe JP. 'Activity of Ciprofloxacin against Multiply Resistant Strains of Pseudomonas aeruginosa, Staphylococcus epidermidis, and Group JK Corynebacteria.' Antimicrobial Agents and Chemotherapy. 1986;Vol 30, No 6:940-941. PMID 3101589.
- ↑ Dreses-Werringloer U, Padubrin I, Jurgens-Saathoff B, Hudson AP, Zeidler H, Kohler L. Persistence of Chlamydia trachomatis Is Induced by Ciprofloxacin and Ofloxacin In Vitro. Antimicrob Agents Chemother 2000 Dec;44(12):3288-97. PMID 11083629
- ↑ Spivey JM, Cummings DM, Pierson NR. Failure of prostatitis treatment secondary to probable ciprofloxacin-sucralfate drug interaction. Pharmacotherapy 1996;16:314-6. PMID 8820479.
- ↑ Drug effects of Ciprofloxacin
- ↑ Fluoroquinolone Resistance in Neisseria gonorrhoeae -- Colorado and Washington, 1995 [1]
- ↑ Schacht P. (1989). Safety of oral ciprofloxacin. An update based on clinical trial results. American Journal of Medicine: Nov 30;87(5A):98S-102S.
- ↑ Rubinstein, E. (2001). History of Quinolones and Their Side Effects. Chemotherapy. 47:3-8 (DOI: 10.1159/000057838)
- ↑ Cipro package insert. Page 7 Warning of tendon ruptures and irreversible neurological conditions.
- ↑ http://www.cbc.ca/health/story/2008/07/08/cipro-fda.html
- ↑ Cipro levaquin side effects floxin avelox
- ↑ Brouwers JR. Drug interactions with quinolone antibacterials. Drug Saf 1992;7:268-81. PMID 1524699.
- ↑ Hilliard JJ, Krause HM, Bernstein JI, Fernandez JA, Nguyen V, Ohemeng KA, Barrett JF. 'A comparison of active site binding of 4-quinolones and novel flavone gyrase inhibitors to DNA gyrase. Adv Exp Med Biol. 1995;390:59-69. PMID 8718602.
External links
- Data sheet for Cipro
- Fluoroquinolone Toxicity Research Foundation querying the overall safety of fluoroquinolones
- Fluoroquinolone-Induced Tendinopathy: What do we know? Richard M. Harrell, MD.
- Emergency Medicine Magzine a review of ciprofloxacin in relation to other fluoroquinolones
- How Stuff Works - Cipro
- MedlinePlus Drug Information: Ciprofloxacin
|
||||||||||||||||||||||