Carbonic acid
| Carbonic acid | |
|---|---|
 |
 |
| Other names | Carbon dioxide solution, dihydrogen carbonate (IUPAC) |
| Identifiers | |
| CAS number | 463-79-6 |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
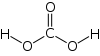
| Molecular formula | H2CO3 |
| Molar mass | 62.03 g/mol |
| Density | 1.0 g/cm3 (dilute solution) |
| Solubility in water | exists only in solution |
| Acidity (pKa) | 6.35 10.25 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Carbonic acid (ancient name acid of air or aerial acid) has the formula H2CO3. It is also a name sometimes given to solutions of carbon dioxide in water, which contain small amounts of H2CO3. The salts of carbonic acids are called bicarbonates (or hydrogencarbonates) and carbonates. It is a weak acid. Carbonic acid should never be confused with carbolic acid, an antiquated name for phenol.
Carbon dioxide dissolved in water is in equilibrium with carbonic acid:
-
- CO2 + H2O ⇌ H2CO3
The hydration equilibrium constant at 25°C is Kh= 1.70×10−3: hence, the majority of the carbon dioxide is not converted into carbonic acid and stays as CO2 molecules. In the absence of a catalyst, the equilibrium is reached quite slowly. The rate constants are 0.039 s−1 for the forward reaction (CO2 + H2O → H2CO3) and 23 s−1 for the reverse reaction (H2CO3 → CO2 + H2O).
Contents |
Role of carbonic acid in blood
Carbonic acid is an intermediate step in the transport of CO2 out of the body via respiratory gas exchange. The hydration reaction of CO2 is generally very slow in the absence of a catalyst, but red blood cells contain carbonic anhydrase which both increases the reaction rate and disassociates a hydrogen ion (H+) from the resulting carbonic acid, leaving bicarbonate (HCO3-) dissolved in the blood plasma. This catalysed reaction is reversed in the lungs, where it converts the bicarbonate back into CO2 and allows it to be expelled.
Carbonic acid also plays a very important role as a buffer in mammalian blood. The equilibrium between carbon dioxide and carbonic acid is very important for controlling the acidity of body fluids, and the carbonic anhydrase increases the reaction rate by a factor of nearly a billion to keep the fluids at a stable pH.
Acidity of carbonic acid
Carbonic acid is diprotic, that is it has two hydrogens which dissociate from the parent molecule, and thus there are two dissociation constants:
-
- H2CO3 ⇌ HCO3− + H+
- Ka1 = 2.5×10−4; pKa1 = 3.60 at 25 °C, for -log (2.5×10−4) = 3.60.
- H2CO3 ⇌ HCO3− + H+
-
- HCO3− ⇌ CO32− + H+
- Ka2 = 5.61×10−11; pKa2 = 10.25 at 25 °C.
- HCO3− ⇌ CO32− + H+
Care must be taken when quoting and using the first dissociation constant of carbonic acid. The value given above is correct for the H2CO3 molecule, and shows that it is a stronger acid than acetic acid or formic acid: this might be expected from the influence of the electronegative oxygen substituent. However, in aqueous solution carbonic acid only exists in equilibrium with carbon dioxide, and the concentration of H2CO3 there is much lower than the CO2 concentration, reducing the measured acidity. The equation may be rewritten as follows (c.f. sulfurous acid):
-
- CO2 + H2O ⇌ HCO3− + H+
- Ka = 4.30×10−7; pKa = 6.36.
- CO2 + H2O ⇌ HCO3− + H+
This figure is quoted as the dissociation constant of carbonic acid, although this is ambiguous: it might better be referred to as the acidity constant of carbon dioxide, as it is particularly useful for calculating the pH of CO2 solutions.
pH and composition of a carbonic acid solution
At a given temperature, the composition of a pure carbonic acid solution (or of a pure CO2 solution) is completely determined by the partial pressure 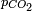 of carbon dioxide above the solution. To calculate this composition, account must be taken of the above equilibria between the three different carbonate forms (H2CO3, HCO3− and CO32−) as well as of the hydration equilibrium between dissolved CO2 and H2CO3 with constant
of carbon dioxide above the solution. To calculate this composition, account must be taken of the above equilibria between the three different carbonate forms (H2CO3, HCO3− and CO32−) as well as of the hydration equilibrium between dissolved CO2 and H2CO3 with constant ![\scriptstyle K_h=\frac{[H_2CO_3]}{[CO_2]}](/2009-wikipedia_en_wp1-0.7_2009-05/I/548164e9dafca2231c39541c7e511fa1.png) (see above) and of the following equilibrium between the dissolved CO2 and the gaseous CO2 above the solution:
(see above) and of the following equilibrium between the dissolved CO2 and the gaseous CO2 above the solution:
- CO2(gas) ↔ CO2(dissolved) with
![\scriptstyle \frac{[CO_2]}{p_{CO_2}}=\frac{1}{k_\mathrm{H}}](/2009-wikipedia_en_wp1-0.7_2009-05/I/f39b32d825fae72e1bdf768f57e48327.png) where kH=29.76 atm/(mol/L) at 25°C (Henry constant)
where kH=29.76 atm/(mol/L) at 25°C (Henry constant)
The corresponding equilibrium equations together with the ![\scriptstyle[H^+][OH^-]=10^{-14}](/2009-wikipedia_en_wp1-0.7_2009-05/I/820d8be050a0a727f6597d89d086a9e9.png) relation and the neutrality condition
relation and the neutrality condition ![\scriptstyle[H^+]=[OH^-]+[HCO_3^-]+2[CO_3^{2-}]](/2009-wikipedia_en_wp1-0.7_2009-05/I/8799cc0efd127b08f6494b6015e9bc97.png) result in six equations for the six unknowns [CO2], [H2CO3], [H+], [OH−], [HCO3−] and [CO32−], showing that the composition of the solution is fully determined by
result in six equations for the six unknowns [CO2], [H2CO3], [H+], [OH−], [HCO3−] and [CO32−], showing that the composition of the solution is fully determined by 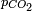 . The equation obtained for [H+] is a cubic whose numerical solution yields the following values for the pH and the different species concentrations:
. The equation obtained for [H+] is a cubic whose numerical solution yields the following values for the pH and the different species concentrations:
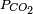 (atm) (atm) |
pH | [CO2] (mol/L) | [H2CO3] (mol/L) | [HCO3−] (mol/L) | [CO32−] (mol/L) |
| 10−8 | 7.00 | 3.36 × 10-10 | 5.71 × 10−13 | 1.42 × 10−9 | 7.90 × 10−13 |
| 10−6 | 6.81 | 3.36 × 10−8 | 5.71 × 10−11 | 9.16 × 10−8 | 3.30 × 10−11 |
| 10−4 | 5.92 | 3.36 × 10−6 | 5.71 × 10−9 | 1.19 × 10−6 | 5.57 × 10−11 |
| 3.5 × 10−4 | 5.65 | 1.18 × 10−5 | 2.00 × 10−8 | 2.23 × 10−6 | 5.60 × 10−11 |
| 10−3 | 5.42 | 3.36 × 10−5 | 5.71 × 10−8 | 3.78 × 10−6 | 5.61 × 10−11 |
| 10−2 | 4.92 | 3.36 × 10−4 | 5.71 × 10−7 | 1.19 × 10−5 | 5.61 × 10−11 |
| 10−1 | 4.42 | 3.36 × 10−3 | 5.71 × 10−6 | 3.78 × 10−5 | 5.61 × 10−11 |
| 1 | 3.92 | 3.36 × 10−2 | 5.71 × 10−5 | 1.20 × 10−4 | 5.61 × 10−11 |
| 2.5 | 3.72 | 8.40 × 10−2 | 1.43 × 10−4 | 1.89 × 10−4 | 5.61 × 10−11 |
| 10 | 3.42 | 0.336 | 5.71 × 10−4 | 3.78 × 10−4 | 5.61 × 10−11 |
- We see that in the total range of pressure, the pH is always largely lower than pKa2 so that the CO32− concentration is always negligible with respect to HCO3− concentration. In fact CO32− play no quantitive role in the present calculation (see remark below).
- For vanishing
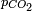 , the pH is close to the one of pure water (pH = 7) and the dissolved carbon is essentially in the HCO3− form.
, the pH is close to the one of pure water (pH = 7) and the dissolved carbon is essentially in the HCO3− form.
- For normal atmospherics conditions (
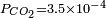 atm), we get a slightly acid solution (pH = 5.7) and the dissolved carbon is now essentially in the CO2 form. From this pressure on, [OH−] becomes also negligible so that the ionized part of the solution is now an equimolar mixture of H+ and HCO3−.
atm), we get a slightly acid solution (pH = 5.7) and the dissolved carbon is now essentially in the CO2 form. From this pressure on, [OH−] becomes also negligible so that the ionized part of the solution is now an equimolar mixture of H+ and HCO3−.
- For a CO2 pressure typical of the one in soda drinks bottles (
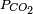 ~ 2.5 atm), we get a relatively acid medium (pH = 3.7) with a high concentration of dissolved CO2. These features contribute to the sour and sparkling taste of these drinks.
~ 2.5 atm), we get a relatively acid medium (pH = 3.7) with a high concentration of dissolved CO2. These features contribute to the sour and sparkling taste of these drinks.
- Between 2.5 and 10 atm, the pH crosses the pKa1 value (3.60) giving a dominant H2CO3 concentration (with respect to HCO3−) at high pressures.
Remark: As noted above, [CO32−] may be neglected for this specific problem, resulting in the following very precise analytical expression for [H+]:
Instability of carbonic acid
It has long been recognized that it is impossible to obtain pure carbonic acid at room temperatures (about 20 °C or about 70 °F). However, in 1991 scientists at NASA's Goddard Space Flight Center (USA) succeeded in making the first pure H2CO3 samples. They did so by exposing a frozen mixture of water and carbon dioxide to high-energy radiation, and then warming to remove the excess water. The carbonic acid that remained was characterized by infrared spectroscopy. The fact that the carbonic acid was prepared by irradiating a solid H2O + CO2 mixture has given rise to suggestions that H2CO3 might be found in outer space, where frozen ices of H2O and CO2 are common, as are cosmic rays and ultraviolet light, to help them react. The same carbonic acid polymorph (denoted beta-carbonic acid) was prepared by a cryotechnique at the University of Innsbruck: alternating layers of glassy aqueous solutions of bicarbonate and acid were heated in vacuo, which causes protonation of bicarbonate, and the solvent was subsequently removed. A second polymorph (denoted alpha-carbonic acid) was prepared by the same technique at the University of Innsbruck using methanol rather than water as a solvent.
It has since been shown, by theoretical calculations, that the presence of even a single molecule of water causes carbonic acid to revert to carbon dioxide and water fairly quickly. Pure carbonic acid is predicted to be stable in the gas phase, in the absence of water, with a calculated half-life of 180,000 years.
There is a hypothetical acid orthocarbonic acid which is even more hydrated, being H4CO4.
Further reading
- Welch, M. J.; Lipton, J. F.; Seck, J. A. (1969). J. Phys. Chem. 73:3351.
- Jolly, W. L. (1991). Modern Inorganic Chemistry (2nd Edn.). New York: McGraw-Hill. ISBN 0-07-112651-1.
- M. H. Moore and R. Khanna "Infrared and Mass Spectral Studies of Proton Irradiated H2O+CO2 Ice: Evidence for Carbonic Acid", Spectrochimica Acta, 47A, pp. 255-262 (1991)
- T. Loerting, C. Tautermann, R.T. Kroemer, I. Kohl, E. Mayer, A. Hallbrucker, K. R. Liedl "On the Surprising Kinetic Stability of Carbonic Acid", Angew. Chem. Int. Ed., 39, pp. 891-895 (2001)
- W. Hage, K. R. Liedl, A. Hallbrucker and E. Mayer, "Carbonic acid in the gas phase and its astrophysical relevance", Science, 279, pp. 1332-1335 (1998)
- W. Hage, A. Hallbrucker, E. Mayer, "Carbonic acid: synthesis by protonation of bicarbonate and FTIR spectroscopic characterization via a new cryogenic technique.", J. Am. Chem. Soc., 115, pp. 8427-8431(1993)
- W. Hage, A. Hallbrucker, E. Mayer, "A polymorph of carbonic acid and its possible astrophysical relevance.", J. Chem. Soc. Farad. Trans., 91, 2823-2826 (1995).
See also
- Carbonated water
- Ocean acidification
- Carbon dioxide
External links
- Ask a Scientist: Carbonic Acid Decomposition
- Why was the existence of carbonic acid unfairly doubted for so long?
|
|||||||||||
![\scriptstyle[H^+] \simeq \left( 10^{-14}+\frac {K_hK_{a1}}{k_\mathrm{H}} p_{CO_2}\right)^{1/2}](/2009-wikipedia_en_wp1-0.7_2009-05/I/831ffd4f77d667186b1d24cf7131235e.png)