Carbon monoxide
| Carbon monoxide | |
|---|---|
 |
 |
| IUPAC name | Carbon monoxide |
| Other names | Carbonic oxide |
| Identifiers | |
| CAS number | 630-08-0 |
| RTECS number | FG3500000 |
| ChemSpider ID | |
| Properties | |
| Molecular formula | CO |
| Molar mass | 28.0101 g/mol |
| Appearance | Colourless, odorless gas |
| Density | 0.789 g/cm³, liquid 1.250 g/L at 0°C, 1 atm. 1.145 g/L at 25°C, 1 atm. (lighter than air) |
| Melting point |
-205 °C (68 K) |
| Boiling point |
-192 °C (81 K) |
| Solubility in water | 0.0026 g/100 mL (20 °C) |
| Dipole moment | 0.112 D (3.74×10−31 C·m) |
| Hazards | |
| EU classification | Highly flammable (F+) Repr. Cat. 1 Toxic (T) |
| NFPA 704 |
 4
4
0
|
| R-phrases | R12, R23, R33, R48, R61 |
| S-phrases | S9, S16, S33, S45, S53 |
| Flash point | Flammable gas |
| Related compounds | |
| Related oxides | carbon dioxide; carbon suboxide; dicarbon monoxide; carbon trioxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Carbon monoxide, with the chemical formula CO, is a colorless, odorless, tasteless yet highly toxic gas. Its molecules consist of one carbon atom covalently bonded to one oxygen atom. There are two covalent bonds and a coordinate covalent bond between the oxygen and carbon atoms.
Carbon monoxide is produced from the partial oxidation of carbon-containing compounds, notably in internal-combustion engines. Carbon monoxide forms in preference to the more usual carbon dioxide when there is a reduced availability of oxygen present during the combustion process. Carbon monoxide has significant fuel value, burning in air with a characteristic blue flame, producing carbon dioxide. Despite its serious toxicity, CO plays a highly useful role in modern technology, being a precursor to myriad products.
Contents |
Production
Carbon monoxide is so fundamentally important that many methods have been developed for its production.[1]
Producer gas is formed by combustion of carbon in oxygen at high temperatures when there is an excess of carbon. In an oven, air is passed through a bed of coke. The initially produced CO2 equilibrates with the remaining hot carbon to give CO. The reaction of O2 with carbon to give CO is described as the Boudouard equilibrium. Above 800 °C, CO is the predominant product:
- O2 + 2 C → 2 CO
- ΔH = -221 kJ/mol
The downside of this method is if done with air it leaves a mixture that is mostly nitrogen.
Synthesis gas or Water gas is produced via the endothermic reaction of steam and carbon:
- H2O + C → H2 + CO
- ΔH = 131 kJ/mol
CO also is a byproduct of the reduction of metal oxide ores with carbon, shown in a simplified form as follows:
- MO + C → M + CO
- ΔH = 131 kJ/mol
Since CO is a gas, the reduction process can be driven by heating, exploiting the positive (favorable) entropy of reaction. The Ellingham diagram shows that CO formation is favored over CO2 in high temperatures.
CO is the anhydride of formic acid. As such it is conveniently produced by the dehydration of formic acid, for example with sulfuric acid. Another laboratory preparation for carbon monoxide entails heating an intimate mixture of powdered zinc metal and calcium carbonate.
Another laboratory method to generate CO is reacting sucrose and sodium hydroxide in a closed system.
Structure
The CO molecule possesses a bond length of 0.1128 nm.[2] Formal charge and electronegativity difference cancel each other out. The result is a small dipole moment with its negative end on the carbon atom[3]. The reason for this, despite oxygen's greater electronegativity, is that the highest occupied molecular orbital has an energy much closer to that of carbon's p orbitals, meaning that greater electron density is found near the carbon. In addition, carbon's lower electronegativity creates a much more diffuse electron cloud, enhancing the dipole moment. This is also the reason that almost all chemistry involving carbon monoxide occurs through the carbon atom, and not the oxygen.
The molecule's bond length is consistent with a partial triple bond. The molecule has a small dipole moment and can be represented by three resonance structures:
Principal chemical reactions
Industrial uses
Carbon monoxide is a major industrial gas that has many applications in bulk chemicals manufacturing.[4]
High volume aldehydes are produced by the hydroformylation reaction of alkenes, CO, and H2. In one of many applications of this technology, hydroformylation is coupled to the Shell Higher Olefin Process to give precursors to detergents.
Methanol is produced by the hydrogenation of CO. In a related reaction, the hydrogenation of CO is coupled to C-C bond formation, as in the Fischer-Tropsch process where CO is hydrogenated to liquid hydrocarbon fuels. This technology allows coal to be converted to petrol.
In the Monsanto process, carbon monoxide and methanol react in the presence of a homogeneous rhodium catalyst and HI to give acetic acid. This process is responsible for most of the industrial production of acetic acid.
Carbon monoxide is a principal component of syngas, which is often used for industrial power. Carbon monoxide(CO) is also used in industrial scale operations for purifying Nickel (Mond Process}.
Coordination chemistry


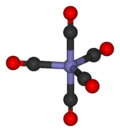
Most metals form coordination complexes containing covalently attached carbon monoxide. Only those in lower oxidation states will complex with carbon monoxide ligands. This is because there must be sufficient electron density to facilitate back donation from the metal dxz-orbital, to the π* molecular orbital from CO. The lone pair on the carbon atom in CO, also donates electron density to the dx²−y² on the metal to form a sigma bond. In nickel carbonyl, Ni(CO)4 forms by the direct combination of carbon monoxide and nickel metal at room temperature. For this reason, nickel in any tubing or part must not come into prolonged contact with carbon monoxide (corrosion). Nickel carbonyl decomposes readily back to Ni and CO upon contact with hot surfaces, and this method was once used for the industrial purification of nickel in the Mond process.[5]
In nickel carbonyl and other carbonyls, the electron pair on the carbon interacts with the metal; the carbon monoxide donates the electron pair to the metal. In these situations, carbon monoxide is called the carbonyl ligand. One of the most important metal carbonyls is iron pentacarbonyl, Fe(CO)5:
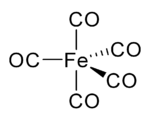
Many metal-CO complexes are prepared by decarbonylation of organic solvents, not from CO. For instance, iridium trichloride and triphenylphosphine react in boiling methoxyethanol or DMF) to afford IrCl(CO)(PPh3)2.
Organic and main group chemistry
In the presence of strong acids and water, carbon monoxide reacts with olefins to form carboxylic acids in a process known as the Koch-Haaf reaction.[6] In the Gattermann-Koch reaction, arenes are converted to benzaldehyde derivatives in the presence of AlCl3 and HCl.[7] Organolithium compounds, e.g. butyl lithium react with CO, but this reaction enjoys little use.
Although CO reacts with carbocations and carbanions, it is relatively unreactive toward organic compounds without the intervention of metal catalysts.[8]
With main group reagents, CO undergoes several noteworthy reactions. Chlorination of CO is the industrial route to the important compound phosgene. With borane CO forms an adduct, H3BCO, which is isoelectronic with the acylium cation [H3CCO]+. CO reacts with sodium to give products resulting from C-C coupling such as Na2C2O2 (sodium acetylenediolate), and potassium to give K2C2O2 (potassium acetylenediolate) and K2C6O6 (potassium rhodizonate).
Carbon monoxide in the atmosphere

Carbon monoxide, though thought of as a pollutant today, has always been present in the atmosphere, chiefly as a product of volcanic activity. It occurs dissolved in molten volcanic rock at high pressures in the earth's mantle. Carbon monoxide contents of volcanic gases vary from less than 0.01% to as much as 2% depending on the volcano. It also occurs naturally in bushfires. Because natural sources of carbon monoxide are so variable from year to year, it is extremely difficult to accurately measure natural emissions of the gas.
Carbon monoxide has an indirect radiative forcing effect by elevating concentrations of methane and tropospheric ozone through chemical reactions with other atmospheric constituents (e.g., the hydroxyl radical, OH.) that would otherwise destroy them. Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide. Carbon monoxide concentrations are both short-lived in the atmosphere and spatially variable.
In urban areas carbon monoxide, along with aldehydes, reacts photochemically to produce peroxy radicals. Peroxy radicals react with nitrogen oxide to increase the ratio of NO2 to NO, which reduces the quantity of NO that is available to react with ozone. Carbon monoxide is also a constituent of tobacco smoke.
Role in physiology and food
Carbon monoxide is used in modified atmosphere packaging systems in the US, mainly with fresh meat products such as beef and pork. The CO combines with myoglobin to form carboxymyoglobin, a bright cherry red pigment. Carboxymyoglobin is more stable than the oxygenated form of myoglobin, oxymyoglobin, which can become oxidized to the brown pigment, metmyoglobin. This stable red colour can persist much longer than in normally packaged meat, giving the appearance of freshness.[9] Typical levels of CO used are 0.4% to 0.5%. The technology was first given generally recognized as safe status by the FDA in 2002 for use as a secondary packaging system. In 2004 the FDA approved CO as primary packaging method, declaring that CO does not mask spoilage odour.[10] Despite this ruling, the technology remains controversial in the US for fears that it is deceptive and masks spoilage.[11] Elsewhere, coloring meat to make it appear fresh is banned in many other countries.
One reaction in the body produces CO. Carbon monoxide is produced naturally as a breakdown of heme (which is one of hemoglobin moieties), a substrate for the enzyme heme oxygenase. The enzymatic reaction results in breakdown of heme to CO, biliverdin and Fe3+ radical. The endogenously produced CO may have important physiological roles in the body (eg as a neurotransmitter or a blood vessels relaxant). In addition CO regulates inflammatory reactions in a manner that prevents the development of several diseases such as atherosclerosis or severe malaria.
CO is a nutrient for methanogenic bacteria,[12] a building block for acetylcoenzyme A. This theme is the subject for the emerging field of bioorganometallic chemistry. In bacteria, CO is produced via the reduction of carbon dioxide via the enzyme carbon monoxide dehydrogenase, an Fe-Ni-S-containing protein.[13]
A haeme-based CO-sensor protein, CooA, is known.[14] The scope of its biological role is still unclear, it is apparently part of a signaling pathway in bacteria and archaea, but its occurrence in mammals is not established.
CO is also currently being studied in several research laboratories throughout the world for its anti-inflammatory and cytoprotective properties that can be used therapeutically to prevent the development of a series of pathologic conditions such as ischemia reperfusion injury, transplant rejection, atherosclerosis, sepsis, severe malaria or autoimmunity. There are yet no clinical applications of CO in humans.
History
It was first described by the Spanish doctor Arnaldus de Villa Nova in the 11th century; but the creation of Carbon monoxide was first made by the French chemist de Lassone in 1776 by heating zinc oxide with coke. He mistakenly concluded that the gaseous product was hydrogen as it burned with a blue flame. The gas was identified as a compound containing carbon and oxygen by the English chemist William Cumberland Cruikshank in the year 1800.
The toxic properties of CO were first thoroughly investigated by the French physiologist Claude Bernard around 1846. He poisoned dogs with the gas, and noticed that their blood was more rutilant in all the vessels. 'Rutilant' is a French word, but also has an entry in English dictionaries, meaning ruddy, shimmering, or golden. However, it was translated at the time as crimson, scarlet, and now is famously known as 'cherry pink'.
During World War II, carbon monoxide was used to keep motor vehicles running in parts of the world where gasoline was scarce. External charcoal or wood burners were fitted, and the carbon monoxide produced by gasification was piped to the carburetor. The CO in this case is known as "wood gas". Carbon monoxide was also reportedly used on a small scale during the Holocaust at some Nazi extermination camps, and in the Action T4 "euthanasia" program.
Source concentrations
- 0.1 ppm - natural background atmosphere level (MOPITT)
- 0.5 to 5 ppm - average background level in homes[15]
- 5 to 15 ppm - levels near properly adjusted gas stoves in homes[15]
- 100-200 ppm - Mexico City central area from autos etc.[16]
- 5,000 ppm - chimney of a home wood fire [17]
- 7,000 ppm - undiluted warm car exhaust - without catalytic converter[17]
- 30,000 ppm - undiluted cigarette smoke[17]
Toxicity
Carbon monoxide is a significantly toxic gas and has no odor or color. It is the most common type of fatal poisoning in many countries.[18] Exposures can lead to significant toxicity of the central nervous system and heart. Following poisoning, long-term sequelae often occurs. Carbon monoxide can also have severe effects on the foetus of a pregnant woman. Symptoms of mild poisoning include headaches and dizziness at concentrations less than 100 ppm. Concentrations as low as 667 ppm can cause up to 50% of the body's haemoglobin to be converted to carboxy-haemoglobin (HbCO). Carboxy-haemoglobin is quite stable but this change is reversible. Carboxy-haemoglobin is ineffective for delivering oxygen, resulting in some body parts not receiving oxygen needed. As a result, exposures of this level can be life-threatening. In the United States, OSHA limits long-term workplace exposure levels to 50 ppm.
The mechanisms by which carbon monoxide produces toxic effects are not yet fully understood, but haemoglobin, myoglobin, and mitochondrial cytochrome oxidase are thought to be compromised. Treatment largely consists of administering 100% oxygen or hyperbaric oxygen therapy, although the optimum treatment remains controversial.[19] Domestic carbon monoxide poisoning can be prevented by the use of household carbon monoxide detectors.
See also
- Carbon monoxide (data page)
- Boudouard reaction
- Carbon monoxide poisoning
- Criteria air contaminants
- Undersea and Hyperbaric Medical Society Hyperbaric Treatment for CO Poisoning
- Rubicon Foundation research articles on CO Poisoning
References
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 200. ISBN 0-12-352651-5.
- ↑ O. R. Gilliam, C. M. Johnson and W. Gordy (1950). "Microwave Spectroscopy in the Region from Two to Three Millimeters". Physical Review 78 (2): 140. doi:.
- ↑ W. Kutzelnigg. Einführung in die Theoretische Chemie. Wiley-VCH. ISBN 3-527-30609-9.
- ↑ Elschenbroich, C.;Salzer, A. ”Organometallics : A Concise Introduction” (2nd Ed) Wiley-VCH: Weinheim, 2006. ISBN 3-527-28165-7
- ↑ Mond L, Langer K, Quincke F (1890). "Action of carbon monoxide on nickel". Journal of the Chemical Society 57: 749–753. doi:.
- ↑ Koch, H.; Haaf, W. (1973). "1-Adamantanecarboxylic Acid". Org. Synth.; Coll. Vol. 5: 20.
- ↑ G. H. Coleman, David Craig (1943). "p-Tolualdehyde". Org. Synth.; Coll. Vol. 2: 583.
- ↑ Chatani, N.; Murai, S. "Carbon Monoxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289
- ↑ Sorheim, S, Nissena, H, Nesbakken, T (1999). "The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide". Journal of Meat Science 52 (2): 157–64. doi:.
- ↑ Eilert EJ (2005). "New packaging technologies for the 21st century". Journal of Meat Science 71 (1): 122–27. doi:.
- ↑ "Low-Oxygen Packaging with CO: A Study in Food Politics That Warrants Peer Review". Retrieved on 2007-04-18.
- ↑ R. K. Thauer (1998). "Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture" (Free). Microbiology 144 (9): 2377–2406. http://mic.sgmjournals.org/cgi/reprint/144/9/2377.
- ↑ Jaouen, G., Ed. (2006). Bioorganometallics: Biomolecules, Labeling, Medicine. Weinheim: Wiley-VCH. ISBN 3-527-30990-X.
- ↑ Roberts, G. P.; Youn, H.; Kerby, R. L. (2004). "CO-Sensing Mechanisms". Microbiology and Molecular Biology Reviews 68: 453–473. doi:. PMID 15353565.
- ↑ 15.0 15.1 "Basic Information : Carbon Monoxide". Retrieved on 2007-12-01.
- ↑ Singer, Siegfried Fred (1975). The Changing Global Environment. pp. 90. ISBN 9789027704023. http://books.google.com/books?id=Ww3DnCF_KZcC&pg=PA90&lpg=PA90&dq=%22carbon+monoxide%22+ppm+concentration+traffic+%22mexico+city%22&source=web&ots=SzIyYgUxWh&sig=ZM4p8whF1mtj1kE3XSx1YUx70zw#PPA90,M1.
- ↑ 17.0 17.1 17.2 Gosink, Tom (1983-01-28). "What Do Carbon Monoxide Levels Mean?" (HTML). Alaska Science Forum. Geophysical Institute, University of Alaska Fairbanks. Retrieved on 2007-12-01.
- ↑ Omaye ST. (2002). "Metabolic modulation of carbon monoxide toxicity". Toxicology 180 (2): 139–50. doi:.
- ↑ Buckley NA, Isbister GK, Stokes B, Juurlink DN. (2005). "Hyperbaric oxygen for carbon monoxide poisoning : a systematic review and critical analysis of the evidence" (Abstract). Toxicol Rev 24 (2): 75–92. doi:. PMID 16180928. http://toxicology.adisonline.com/pt/re/tox/abstract.00139709-200524020-00002.htm.
External links
- International Chemical Safety Card 0023
- National Pollutant Inventory - Carbon Monoxide
- NIOSH Pocket Guide to Chemical Hazards
- CID 281 from PubChem
- External MSDS data sheet
- Carbon Monoxide Kills Awareness Campaign Site
- Carbon Monoxide Purification Process
- Carbon Monoxide Hazards with Backpacking Stoves
- USFDA IMPORT BULLETIN 16B-95, May 1999
- FDA Agency Response Letter GRAS Notice No. GRN 000083
- Carbon Monoxide in Fresh Meat site
- Carbon Monoxide Network & Forum
- Microscale Gas Chemistry Experiments with Carbon Monoxide
- Research on the therapeutic effects of CO (Gulbenkian Science Institute)
- Instant insight outlining the physiology of carbon monoxide from the Royal Society of Chemistry
- [1] Article about Sen. Chris mandating CO detectors in new homes & hotels in Florida as of 2008.
|
|||||||||||