Carbon disulfide
| Carbon disulfide | |
|---|---|
 |
|
 |
|
| IUPAC name | Carbon disulfide |
| Other names | Dithiocarbonic anhydride |
| Identifiers | |
| CAS number | 75-15-0 |
| SMILES |
|
| Properties | |
| Molecular formula | CS2 |
| Molar mass | 76.1 g/mol |
| Appearance | colorless liquid impure: light-yellow |
| Density | 1.26 g/cm³ |
| Melting point |
-111.6 °C (161.6 K) |
| Boiling point |
46 °C (319 K) |
| Solubility in other solvents | 0.2 g/100 ml of water (20 °C) |
| Hazards | |
| NFPA 704 |
 4
3
0
|
| R-phrases | R11, R23, R24, R25, R48 |
| S-phrases | S16, S33, , S53 |
| Flash point | -30 °C |
| Autoignition temperature |
90 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Carbon disulfide is a colorless, volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent. It has an "ether-like" odor, but commercial samples are typically contaminated with foul-smelling impurities, such as carbonyl sulfide[1].
Contents |
Occurrence and manufacture
Small amounts of carbon disulfide are released by volcanic eruptions and marshes. CS2 once was manufactured by combining carbon (or coke) and sulfur at high temperatures. A lower temperature reaction, requiring only 600 °C utilizes natural gas as the carbon source in the presence of silica gel or alumina catalysts:[1]
- CH4 + ½S8 → CS2 + 2H2S
The reaction is analogous to the combustion of methane. Although it is isoelectronic to carbon dioxide, CS2 is highly flammable:
- CS2 + 3O2 → CO2 + 2SO2
Reactions
Compared to CO2, CS2 is more reactive toward nucleophiles and more easily reduced. These differences in reactivity can be attributed to the weaker π donor-ability of the sulfido centers, which renders the carbon more electrophilic. It is widely used in the synthesis of organosulfur compounds such as metham sodium, a soil fumigant and is commonly used in the production of the soft fabric viscose.
Addition of nucleophiles
Nucleophiles such as amines afford dithiocarbamates:
- 2R2NH + CS2 → [R2NH2+][R2NCS2−]
Xanthates form similarly from alkoxides:
- RONa + CS2 → [Na+][ROCS2−]
This reaction is the basis of the manufacture of regenerated cellulose, the main ingredient of viscose, rayon and cellophane. Both xanthates and the related thioxanthates (derived from treatment of CS2 with sodium thiolates) are used as flotation agents in mineral processing.
Sodium sulfide affords trithiocarbonate:
- Na2S + CS2 → [Na+]2[CS32−]
Reduction
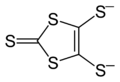
Sodium reduces CS2 to give the heterocycle "dmit2−":[2]
-
- 3CS2 + 4Na → Na2C3S5 + Na2S
Direct electrochemical reduction affords the tetrathiooxalate anion:[3]
-
- 2CS2 + 2e− → C2S42−
Chlorination
Chlorination of CS2 is the principal route to carbon tetrachloride:[1]
- CS2 + 3Cl2 → CCl4 + S2Cl2
This conversion proceeds via the intermediacy of thiophosgene, CSCl2.
Coordination chemistry
CS2 is a ligand for many metal complexes, forming pi complexes. One example is CpCo(η2-CS2)(PMe3).[4]
Commercial Availability
CS2, being highly flammable and having one of the lowest autoignition temperatures, cannot be transported easily using commercial means. Worldwide exports of this chemical are negligible.
Pressurized Liquid Nitrogen Based Sample
Johnson Matthey's sister company Alfa Aesar was the first company to introduce carbon disulfide in the form of pressurized bottle containing a solution of pressurized nitrogen, coupling agent, stablizer, and carbon disulfide, with an active carbon disulfide content of 85%. Dilution with nitrogen rendered contents nonflammable. In 2007 Alfa Aesar stopped selling carbon disulfide samples.
Health effects
At very high levels, carbon disulfide may be life-threatening because it affects the nervous system. Significant safety data come from the viscose rayon Industry, where both carbon disulfide as well as small amounts of H2S may be present.
References
- ↑ 1.0 1.1 1.2 Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA, 1999.ISBN: 0935702482
- ↑ Jeroschewski, P. "Electrochemical Preparation of Tetraalkylammonium Salts of Tetrathiooxalic Acid" Zeitschrift für Chemie (1981), volume 21, 412.
- ↑ Werner, H. (1982). "Novel Coordination Compounds formed from CS2 and Heteroallenes". Coordination Chemistry Reviews 43: 165–185. doi:.