Radiocarbon dating
Radiocarbon dating is a radiometric dating method that uses the naturally occurring radioisotope carbon-14 (14C) to determine the age of carbonaceous materials up to about 60,000 years.[1] Raw, i.e. uncalibrated, radiocarbon ages are usually reported in radiocarbon years "Before Present" (BP), "Present" being defined as AD 1950. Such raw ages can be calibrated to give calendar dates.
One of the most frequent uses of radiocarbon dating is to estimate the age of organic remains from archaeological sites. When plants fix atmospheric carbon dioxide (CO2) into organic material during photosynthesis they incorporate a quantity of 14C that approximately matches the level of this isotope in the atmosphere (a small difference occurs because of isotope fractionation, but this is corrected after laboratory analysis). After plants die or they are consumed by other organisms (for example, by humans or other animals) the 14C fraction of this organic material declines at a fixed exponential rate due to the radioactive decay of 14C. Comparing the remaining 14C fraction of a sample to that expected from atmospheric 14C allows the age of the sample to be estimated.
The technique of radiocarbon dating was developed by Willard Libby and his colleagues at the University of Chicago in 1949.[2] Libby estimated that the steady state radioactivity concentration of exchangeable carbon-14 would be about 14 disintegrations per minute (dpm) per gram. In 1960, he was awarded the Nobel Prize in chemistry for this work. He first demonstrated the accuracy of radiocarbon dating by accurately measuring the age of wood from an ancient Egyptian royal barge whose age was known from historical documents.[2]
Contents |
Basic physics

Carbon has two stable, nonradioactive isotopes: carbon-12 (12C), and carbon-13 (13C). In addition, there are trace amounts of the unstable isotope carbon-14 (14C) on Earth. Carbon-14 has a half-life of 5730 years and would have long ago vanished from Earth were it not for the unremitting cosmic ray impacts on nitrogen in the Earth's atmosphere, which create more of the isotope. The neutrons resulting from the cosmic ray interactions participate in the following nuclear reaction on the atoms of nitrogen molecules (N2) in the atmosphere:
The highest rate of carbon-14 production takes place at altitudes of 9 to 15 km (30,000 to 50,000 ft), and at high geomagnetic latitudes, but the carbon-14 spreads evenly throughout the atmosphere and reacts with oxygen to form carbon dioxide. Carbon dioxide also permeates the oceans, dissolving in the water. For approximate analysis it is assumed that the cosmic ray flux is constant over long periods of time; thus carbon-14 is produced at a constant rate and the proportion of radioactive to non-radioactive carbon is constant: ca. 1 part per trillion (600 billion atoms/mole). In 1958 Hessel de Vries showed that the concentration of carbon-14 in the atmosphere varies with time and locality. For the most accurate work, these variations are compensated by means of calibration curves. When these curves are used, their accuracy and shape are the factors that determine the accuracy and age obtained for a given sample.
Plants take up atmospheric carbon dioxide by photosynthesis, and are ingested by animals, so every living thing is constantly exchanging carbon-14 with its environment as long as it lives. Once it dies, however, this exchange stops, and the amount of carbon-14 gradually decreases through radioactive beta decay.
Computation of ages and dates
The radioactive decay of carbon-14 follows an exponential decay. A quantity is said to be subject to exponential decay if it decreases at a rate proportional to its value. Symbolically, this can be expressed as the following differential equation, where N is the quantity and λ is a positive number called the decay constant:
The solution to this equation is:
 ,
,
where, for a given sample of carbonaceous matter:
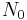 = number of radiocarbon atoms at
= number of radiocarbon atoms at 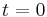 , i.e. the origin of the disintegration time,
, i.e. the origin of the disintegration time, = number of radiocarbon atoms remaining after radioactive decay during the time
= number of radiocarbon atoms remaining after radioactive decay during the time 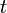 ,
,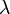 = radiocarbon decay or disintegration constant.
= radiocarbon decay or disintegration constant.
Two related times can be defined:
-
- mean- or average-life: mean or average time each radiocarbon atom spends in a given sample until it decays.
- half-life: time lapsed for half the number of radiocarbon atoms in a given sample, to decay,
It can be shown that:
 =
=  = radiocarbon mean- or average-life = 8033 years (Libby value)
= radiocarbon mean- or average-life = 8033 years (Libby value)
 =
=  = radiocarbon half-life = 5568 years (Libby value)
= radiocarbon half-life = 5568 years (Libby value)
Notice that dates are customarily given in years BP which implies t(BP) = -t because the time arrow for dates runs in reverse direction from the time arrow for the corresponding ages. From these considerations and the above equation, it results:
For a raw radiocarbon date:
and for a raw radiocarbon age:
After replacing values, the raw radiocarbon age becomes any of the following equivalent formulae:
using logs base e and the average life:
and
using logs base 2 and the half-life:
Measurements and scales
Measurements are traditionally made by counting the radioactive decay of individual carbon atoms by gas proportional counting or by liquid scintillation counting. For samples of sufficient size (several grams of carbon) this method is still widely used in the 2000s. Among others, all the tree ring samples used for the calibration curves (see below) were determined by these counting techniques. Such decay counting, however, is relatively insensitive and subject to large statistical uncertainties for small samples. When there is little carbon-14 to begin with, the long radiocarbon half-life means that very few of the carbon-14 atoms will decay during the time allotted for their detection, resulting in few disintegrations per minute.
The sensitivity of the method has been greatly increased by the use of Accelerator Mass Spectrometry (AMS). With this technique 14C atoms can be detected and counted directly vs only detecting those atoms that decay during the time interval allotted for an analysis. AMS allows dating samples containing only a few milligrams of carbon.
Raw radiocarbon ages (i.e., those not calibrated) are usually reported in "years Before Present" (BP). This is the number of radiocarbon years before 1950, based on a nominal (and assumed constant - see "calibration" below) level of carbon-14 in the atmosphere equal to the 1950 level. These raw dates are also based on a slightly-off historic value for the radiocarbon half-life. Such value is used for consistency with earlier published dates (see "Radiocarbon half-life" below). See the section on computation for the basis of the calculations.
Radiocarbon dating laboratories generally report an uncertainty for each date. For example, 3000±30BP indicates a standard deviation of 30 radiocarbon years. Traditionally this included only the statistical counting uncertainty. However, some laboratories supplied an "error multiplier" that could be multiplied by the uncertainty to account for other sources of error in the measuring process. More recently, the laboratories try to quote the overall uncertainty, which is determined from control samples of known age and verified by international intercomparison exercises [6]. In 2008, a typical uncertainty better than ±40 radiocarbon years can be expected for samples younger than 10,000 years. This, however, is only a small part of the uncertainty of the final age determination (see section Calibration below).
As of 2007[update], the limiting age for a 1 milligram sample of graphite is about ten half-lives, approximately 60,000 years[7]. This age is derived from that of the calibration blanks used in an analysis, whose 14C content is assumed to be the result of contamination during processing (as a result of this, some facilities[7] will not report an age greater than 60,000 years for any sample).
A variety of sample processing and instrument-based constraints have been postulated to explain the upper age-limit. To examine instrument-based background activities in the AMS instrument of the W. M. Keck Carbon Cycle Accelerator Mass Spectrometry Laboratory of the University of California, a set of natural diamonds were dated. Natural diamond samples from different sources within rock formations with standard geological ages in excess of 100 my yielded 14C apparent ages 64,920±430 BP to 80,000±1100 BP as reported in 2007[8].
Calibration
The need for calibration

A raw BP date cannot be used directly as a calendar date, because the level of atmospheric 14C has not been strictly constant during the span of time that can be radiocarbon dated. The level is affected by variations in the cosmic ray intensity which is in turn affected by variations in the earth's magnetosphere. In addition, there are substantial reservoirs of carbon in organic matter, the ocean, ocean sediments (see methane hydrate), and sedimentary rocks. Changes in the Earth's climate can affect the carbon flows between these reservoirs and the atmosphere, leading to changes in the atmosphere's 14C fraction.
Aside from these changes due to natural processes, the level has also been affected by human activities. From the beginning of the industrial revolution in the 18th century to the 1950s, the fractional level of 14C decreased because of the admixture of large quantities of CO2 into the atmosphere, the combustion production of fossil fuel. This decline is known as the Suess effect, and also affects the 13C isotope. However, atmospheric 14C was almost doubled for a short period during the 1950s and 1960s due to atomic bomb tests.
Calibration methods
The raw radiocarbon dates, in BP years, are calibrated to give calendar dates. Standard calibration curves are available, based on comparison of radiocarbon dates of samples that can be dated independently by other methods such as examination of tree growth rings (dendrochronology), deep ocean sediment cores, lake sediment varves, coral samples, and speleothems (cave deposits).
The calibration curves can vary significantly from a straight line, so comparison of uncalibrated radiocarbon dates (e.g., plotting them on a graph or subtracting dates to give elapsed time) is likely to give misleading results. There are also significant plateaus in the curves, such as the one from 11,000 to 10,000 radiocarbon years BP, which is believed to be associated with changing ocean circulation during the Younger Dryas period. Over the historical period from 0 to 10,000 years BP, the average width of the uncertainty of calibrated dates was found to be 335 years, although in well-behaved regions of the calibration curve the width decreased to about 113 years while in ill-behaved regions it increased to a maximum of 801 years. Significantly, in the ill-behaved regions of the calibration curve, increasing the precision of the measurements does not have a significant effect on increasing the accuracy of the dates.[10]
The 2004 version of the calibration curve extends back quite accurately to 26,000 years BP. Any errors in the calibration curve do not contribute more than ±16 years to the measurement error during the historic and late prehistoric periods (0 - 6,000 yrs BP) and no more than ±163 years over the entire 26,000 years of the curve, although its shape can reduce the accuracy as mentioned above.[11]
Radiocarbon half-life
Libby vs Cambridge values
Carbon dating was developed by a team led by Willard Libby. He worked out a carbon-14 half-life of 5568±30 years, the Libby half-life. Later a more accurate figure of 5730±40 years was determined, which is known as the Cambridge half-life. This is, however, not relevant for radiocarbon dating. If calibration is applied, the half-life cancels out, as long as the same value is used throughout the calculations. Laboratories continue to use the Libby figure to avoid inconsistencies with previous publications.
Carbon exchange reservoir
Libby's original exchange reservoir hypothesis assumes that the exchange reservoir is constant all over the world. The calibration method also assumes that the temporal variation in 14C level is global, such that a small number of samples from a specific year are sufficient for calibration.[12] However, since Libby's early work was published (1950 to 1958), latitudinal and continental variations in the carbon exchange reservoir have been observed by Hessel de Vries (1958; as reviewed by Lerman et al., 1959, 1960). Subsequently, methods have been developed that allow the correction of these so-called reservoir effects, including:
- When CO2 is transferred from the atmosphere to the oceans, it initially shares the 14C concentration of the atmosphere. However, turnaround times of CO2 in the ocean are similar to the half-life of 14C (making 14C also a dating tool for ocean water[13]. Marine organisms feed on this "old" carbon, and thus their radiocarbon age reflects the time of CO2 uptake by the ocean rather than the time of death of the organism. This marine reservoir effect is partly handled by a special marine calibration curve [14], but local deviation of several 100 years exist.
- Erosion and immersion of carbonate rocks (which are generally older than 80,000 years and so shouldn't contain measurable 14C) causes an increase in 12C and 13C in the exchange reservoir, which depends on local weather conditions and can vary the ratio of carbon that living organisms incorporate. This is believed negligible for the atmosphere and atmosphere-derived carbon since most erosion will flow into the sea.[15] The atmospheric 14C concentration may differ substantially from the concentration in local water reservoirs. Eroded from CaCO3 or organic deposits, old carbon may be assimilated easily and provide diluted 14C carbon into trophic chains. So the method is less reliable for such materials as well as for samples derived from animals with such plants in their food chain.
- Volcanic eruptions eject large amount of carbonate into the air, causing an increase in 12C and 13C in the exchange reservoir and can vary the exchange ratio locally. This explains the often irregular dating achieved in volcanic areas.[15]
- The earth is not affected evenly by cosmic radiation, the magnitude of the radiation depends on land altitude and earth's magnetic field strength at any given location, causing minor variation in the local 14C production. This is accounted for by having calibration curves for different locations of the globe. However this could not always be performed, as tree rings for calibration were only recoverable from certain locations in 1958.[16] The rebuttals by Münnich et al.[17] and by Barker[18] both maintain that while variations of carbon-14 exist, they are about an order of magnitude smaller than those implied by Crowe's calculations.
These effects were first confirmed when samples of wood from around the world, which all had the same age (based on tree ring analysis), showed deviations from the dendrochronological age. Calibration techniques based on tree-ring samples have contributed to increase the accuracy since 1962, when they were accurate to 700 years at worst.[19]
Speleothem studies extend 14C calibration
Relatively recent (2001) evidence has allowed scientists to refine the knowledge of one of the underlying assumptions. A peak in the amount of carbon-14 was discovered by scientists studying speleothems in caves in the Bahamas. Stalagmites are calcium carbonate deposits left behind when seepage water, containing dissolved carbon dioxide, evaporates. Carbon-14 levels were found to be twice as high as modern levels.[20] These discoveries improved the calibration for the radiocarbon technique and extended its usefulness to 45,000 years into the past.[21]
Examples
- Ancient footprints of Acahualinca
- Chauvet Cave
- Dolaucothi
- Haraldskær Woman
- Kennewick Man
- Skeleton Lake
- Shroud of Turin
- Thera eruption
- Vinland map
See also
- Age of the Earth
- Cosmogenic isotopes
- Environmental isotopes
- Discussion of half-life and average-life or mean-lifetime
References
- ↑ Plastino, W.; Kaihola, L.; Bartolomei, P.; Bella, F. (2001). "Cosmic Background Reduction In The Radiocarbon Measurement By Scintillation Spectrometry At The Underground Laboratory Of Gran Sasso". Radiocarbon 43 (2A): 157–161. https://digitalcommons.library.arizona.edu/objectviewer?o=http%3A%2F%2Fradiocarbon.library.arizona.edu%2Fvolume43%2Fnumber2A%2Fazu_radiocarbon_v43_n2a_157_161_v.pdf.
- ↑ 2.0 2.1 Arnold, J. R.; Libby, W. F. (1949). "Age Determinations by Radiocarbon Content: Checks with Samples of Known Age". Science 110 (2869): 678–680. doi:. PMID 15407879. http://hbar.phys.msu.ru/gorm/fomenko/libby.htm.
- ↑ "Atmospheric δ14C record from Wellington". Carbon Dioxide Information Analysis Center. Retrieved on 1 May 2008.
- ↑ "δ14CO2 record from Vermunt". Carbon Dioxide Information Analysis Center. Retrieved on 1 May 2008.
- ↑ "Radiocarbon dating". Utrecht University. Retrieved on 1 May 2008.
- ↑ Scott, EM (2003). "The Fourth International Radiocarbon Intercomparison (FIRI).". Radiocarbon 45: 135–285.
- ↑ 7.0 7.1 "NOSAMS Radiocarbon Data and Calculations". Woods Hole Oceanographic Institution.
- ↑ Taylor RE, Southon J (2007). "Use of natural diamonds to monitor 14C AMS instrument backgrounds". Nuclear Instruments and Methods in Physics Research B 259: 282–28. doi:.
- ↑ Stuiver M, Reimer PJ, Braziunas TF (1998). "High-precision radiocarbon age calibration for terrestrial and marine samples". Radiocarbon 40: 1127–51. http://depts.washington.edu/qil/datasets/uwten98_14c.txt.
- ↑ These results were obtained from a Monte Carlo analysis calibrating simulated measurements of varying precision using the 1993 version of the calibration curve. The width of the uncertainty represents a 2σ uncertainty (that is, a likelihood of 95% that the date appears between these limits). Niklaus TR, Bonani G, Suter M, Wölfli W (1994). "Systematic investigation of uncertainties in radiocarbon dating due to fluctuations in the calibration curve". Nuclear Instruments and Methods in Physics Research 92: 194–200.
- ↑ Reimer Paula J et al. (2004). "INTCAL04 Terrestrial Radiocarbon Age Calibration, 0–26 Cal Kyr BP". Radiocarbon 46 (3): 1029–1058. http://digitalcommons.library.arizona.edu/index.php/objectviewer?o=http://radiocarbon.library.arizona.edu/Volume46/Number3/azu_radiocarbon_v46_n3_1029_1058_v.pdf.
- ↑ Libby WF (1955). Radiocarbon dating (2nd edition ed.). Chicago: University of Chicago Press.
- ↑ McNichol AP, Schneider RJ, von Reden KF, Gagnon AR, Elder KL, NOSAMS, Key RM, Quay PD (October 2000). "Ten years after - The WOCE AMS radiocarbon program". Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms 172 (1-4): 479–84. http://www.sciencedirect.com/science/article/B6TJN-41MHFHC-2Y/1/d7be7f3719c79ac70a3ab99ca1bc97c6.
- ↑ Stuiver M, Braziunas TF (1993). "Modelling atmospheric 14C influences and 14C ages of marine samples to 10,000 BC". Radiocarbon 35 (1): 137.
- ↑ 15.0 15.1 Kolchin BA, Shez YA (1972). Absolute archaeological datings and their problems. Moscow: Nauka.
- ↑ Crowe C (1958). "Carbon-14 activity during the past 5000 years". Nature 182: 470–1. http://www.nature.com/nature/journal/v182/n4633/abs/182470a0.html.
- ↑ Münnich KO, Östlund HG, de Vries H (1958). "Carbon-14 Activity during the past 5,000 Years". Nature 182: 1432–3. http://www.nature.com/nature/journal/v182/n4647/abs/1821432a0.html.
- ↑ Barker H (1958). "Carbon-14 Activity during the past 5,000 Years". Nature 182: 1433. http://www.nature.com/nature/journal/v182/n4647/abs/1821433a0.html.
- ↑ Libby WF (1962). "Radiocarbon; an atomic clock". Annual Science and Humanity Journal.
- ↑ Pennicott K (10 May 2001). "Carbon clock could show the wrong time". PhysicsWeb.
- ↑ Jensen MN (2001). "Peering deep into the past". University of Arizona, Department of Physics.
Further reading
- Bowman, Sheridan (1990). Interpreting the Past: Radiocarbon Dating. Berkeley: University of California Press. ISBN 0520070372.
- Currie, L. (2004). "The Remarkable Metrological History of Radiocarbon Dating II". J. Res. Natl. Inst. Stand. Technol. 109: 185–217. http://nvl.nist.gov/pub/nistpubs/jres/109/2/j92cur.pdf.
- de Vries, H. L. (1958). "Variation in Concentration of Radiocarbon with Time and Location on Earth", Proceedings Koninlijke Nederlandse Akademie Wetenschappen B, 61: 94-102; and in Researches in Geochemistry, P. H. Abelson (Ed.) (1959) Wiley, New York, p. 180.
- Friedrich, M.; et al. (2004). "The 12,460-Year Hohenheim Oak and Pine Tree-Ring Chronology from Central Europe—a Unique Annual Record for Radiocarbon Calibration and Paleoenvironment Reconstructions". Radiocarbon 46: 1111–1122.
- Gove, H. E. (1999) From Hiroshima to the Iceman. The Development and Applications of Accelerator Mass Spectrometry. Bristol: Institute of Physics Publishing.
- Kovar, Anton J. (1966). "Problems in Radiocarbon Dating at Teotihuacan". American Antiquity 31: 427–430. doi:. http://www.jstor.org/pss/2694748.
- Lerman, J. C.; Mook, W. G.; Vogel, J. C.; de Waard, H. (1969). "Carbon-14 in Patagonian Tree Rings". Science 165 (3898): 1123–1125. doi:. PMID 17779805.; Lerman, J. C., Mook, W. G., and Vogel, J. C. (1970) Proc. 12th Nobel Symp.
- Lorenz, R. D.; Jull, A. J. T.; Lunine, J. I.; Swindle, T. (2002). "Radiocarbon on Titan". Meteoritics and Planetary Science 37: 867–874.
- Mook, W. G.; van der Plicht, J. (1999). "Reporting 14C activities and concentrations". Radiocarbon 41: 227–239. http://digitalcommons.library.arizona.edu/index.php/objectviewer?o=http://radiocarbon.library.arizona.edu/Volume41/Number3/azu_radiocarbon_v41_n3_227_239_v.pdf.
- Weart, S. (2004) The Discovery of Global Warming - Uses of Radiocarbon Dating.
- Willis, E.H. (1996) Radiocarbon dating in Cambridge: some personal recollections. A Worm's Eye View of the Early Days.
External links
- Radiocarbon - The main international journal of record for research articles and date lists relevant to 14C
- C14dating.com - General information on Radiocarbon dating
- calib.org - Calibration program, Marine Reservoir database, and bomb calibration
- NOSAMS: National Ocean Sciences Accelerator Mass Spectrometry Facility at the Woods Hole Oceanographic Institution
- Discussion of calibration (from U Oxford)
- Several calibration programs can be found at www.radiocarbon.org
- CalPal Online (Cologne Radiocarbon Calibration & Paleoclimate Research Package)
- OxCal program (Oxford Calibration)
- Fairbanks' Radiocarbon Calibration program (for prior to 12400 BP)
- Notes on radiocarbon dating, including movies illustrating the atomic physics (from UC Santa Barbara)
|
||||||||||||||||||||||||||||







