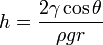Capillary action

Capillary action, capillarity, capillary motion, or wicking is the ability of a substance to draw another substance into it. The standard reference of capillary action is to a tube in plants, but it can be seen readily with porous paper. It occurs when the adhesive intermolecular forces between the liquid and a substance are stronger than the cohesive intermolecular forces inside the liquid. The effect causes a concave meniscus to form where the substance is touching a vertical surface. The same effect is what causes porous materials such as sponges to soak up liquids.
A flow of carrier gas in a silica capillary column of a GC system. This flow can be calculated by Poiseuille's equation for compressible fluids.
Contents |
Examples
In hydrology, capillary action describes the attraction of water molecules to soil particles. Capillary action is responsible for moving groundwater from wet areas of the soil to dry areas. Differences in soil potential (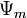 ) drive capillary action in soil.
) drive capillary action in soil.
Capillary action is also essential for the drainage of constantly produced tear fluid from the eye. Two canalicula of tiny diameter are present in the inner corner of the eyelid, also called the lacrymal ducts; their openings can be seen with the naked eye within the lacrymal sacs when the eyelids are everted.
Paper towels absorb liquid through capillary action, allowing a fluid to be transferred from a surface to the towel. The small pores of a sponge act as small capillaries, causing it to absorb a comparatively large amount of fluid.
Some old sport and exercise fabrics, such as Coolmax, use capillary action to "wick" sweat away from the skin. These are often referred to as wicking fabrics, presumably after the capillary properties of a candle wick.
Chemists utilize capillary action in thin layer chromatography, in which a solvent moves vertically up a plate via capillary action. Dissolved solutes travel with the solvent at various speeds depending on their polarity.
Capillary action is NOT responsible for water transport in plants. Instead cohesion between the water molecules and transpiration work together to draw up water.
Formula

The height h of a liquid column is given by:[1]
where:
-
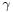 is the liquid-air surface tension (energy/area)
is the liquid-air surface tension (energy/area)- θ is the contact angle
- ρ is the density of liquid (mass/volume)
- g is acceleration due to gravity (length/time2)
- r is radius of tube (length).
For a water-filled glass tube in air at sea level, using SI units:
therefore, the height of the water column is given by:
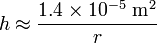 .
.
Thus for a 2 m wide (1 m radius) tube, the water would rise an unnoticeable 0.014 mm. However, for a 2 cm wide (0.01 m radius) tube, the water would rise 1.4 mm, and for a 0.2 mm wide (0.0001 m radius) tube, the water would rise 140 mm (about 5.5 inches).
Miscellaneous
Albert Einstein's first paper[2] submitted to Annalen der Physik was on capillarity. It was titled Folgerungen aus den Capillaritätserscheinungen, which translates as Conclusions from the capillarity phenomena, found in volume 4, page 513.[3] It was submitted in late 1900 and was published in 1901. In 1905 Einstein published four seminal papers in the same journal; these four papers are known as the Annus Mirabilis Papers.
See also
- Frost flowers
- Washburn's equation
- Wick effect
- Capillary fringe
- Capillary wave
References
- ↑ G.K. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Press (1967) ISBN 0521663962
- ↑ List of Scientific Publications of Albert Einstein
- ↑ Folgerungen aus den Capillaritätserscheinungen (in German)