Cannabis
| Cannabis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
 common hemp
|
||||||||||||
| Scientific classification | ||||||||||||
|
||||||||||||
| Species | ||||||||||||
|
Cannabis sativa subsp. indica L.[1] |
Cannabis (Cán-na-bis) is a genus of flowering plants that includes three putative species, Cannabis sativa subsp. indica L.,[1] Cannabis sativa subsp. sativa Lam.,[1] and Cannabis ruderalis Janisch. These three taxa are indigenous to central Asia and surrounding regions. Cannabis has long been used for fibre (hemp), for medicinal purposes, and as a psychoactive. Industrial hemp products are made from Cannabis plants selected to produce an abundance of fiber and minimal levels of THC (Δ9- tetrahydrocannabinol), one psychoactive molecule that produces the "high" associated with marijuana. The drug consists of dried flowers and leaves of plants selected to produce high levels of THC. Various extracts including hashish and hash oil are also produced.[2] The cultivation and possession of Cannabis for recreational use is outlawed in most countries.
Contents |
Etymology
The plant name cannabis is from Greek κάνναβις (kánnabis), via Latin cannabis, a Scythian or Thracian word, also loaned into Persian as kanab. English hemp (Old English hænep) may be an early loan (predating Grimm's Law) from the same source. In Hebrew, the word is קַנַּבּוֹס [qan:a'bos]. Old Akkadian qunnabtu, Neo-Assyrian and Neo-Babylonian qunnabu were used to refer to the plant.[3][4]
The further origin of the Scythian term is uncertain.
Description
Cannabis is an annual, dioecious, flowering herb. The leaves are palmately compound, with serrate leaflets. The first pair of leaves usually have a single leaflet, the number gradually increasing up to a maximum of about thirteen leaflets per leaf (usually seven or nine), depending on variety and growing conditions. At the top of a flowering plant, this number again diminishes to a single leaflet per leaf. The lower leaf pairs usually occur in an opposite leaf arrangement and the upper leaf pairs in an alternate arrangement on the main stem of a mature plant.
Cannabis usually has imperfect flowers with staminate "male" and pistillate "female" flowers occurring on separate plants,[5] although hermaphroditic plants sometimes occur.[6] Male flowers are borne on loose panicles, and female flowers are borne on racemes.[7] It is not unusual for individual plants to bear both male and female flowers, though these are referred to as 'intersexual' or hermaphroditic rather than monoecious, since staminate and pistillate structures appear at different points on the plant, not within the same flower.
Cannabinoids, terpenoids, and other compounds are secreted by glandular trichomes that occur most abundantly on the floral calyxes and bracts of female plants.[8]
All known strains of Cannabis are wind-pollinated[9] and produce "seeds" that are technically called achenes.[10] Most strains of Cannabis are short day plants,[9] with the possible exception of C. sativa subsp. sativa var. spontanea (= C. ruderalis), which is commonly described as "auto-flowering" and may be day-neutral.
Cannabis is naturally diploid, having a chromosome complement of 2n=20, although polyploid individuals have been artificially produced.[11] Cannabis is a genus of flowering plant which includes one or more species. The plant is believed to have originated in the mountainous regions just north west of the Himalayas. It is also known as hemp, although this term usually refers to varieties of Cannabis cultivated for non-drug use. Cannabis plants produce a group of chemicals called cannabinoids which produce mental and physical effects when consumed. As a drug it usually comes in the form of dried buds or flowers (marijuana), resin (hashish), or various extracts collectively known as hashish oil.[2] In the early 20th century, it became illegal in most of the world to cultivate or possess Cannabis for drug purposes.
Taxonomy

The genus Cannabis was formerly placed in the Nettle (Urticaceae) or Mulberry (Moraceae) family, but is now considered along with hops (Humulus sp.) to belong to the Hemp family (Cannabaceae).[12] Recent phylogenetic studies based on cpDNA restriction site analysis and gene sequencing strongly suggest that the Cannabaceae arose from within the Celtidaceae clade, and that the two families should be merged to form a single monophyletic group.[13][14]
Various types of Cannabis have been described, and classified as species, subspecies, or varieties:[15]
- plants cultivated for fiber and seed production, described as low-intoxicant, non-drug, or fiber types
- plants cultivated for drug production, described as high-intoxicant or drug types
- escaped or wild forms of either of the above types.
Cannabis plants produce a unique family of terpeno-phenolic compounds called cannabinoids, which produce the "high" one experiences from smoking marijuana. The two cannabinoids usually produced in greatest abundance are cannabidiol (CBD) and/or Δ9-tetrahydrocannabinol (THC), but only THC is psychoactive. Since the early 1970s, Cannabis plants have been categorized by their chemical phenotype or "chemotype," based on the overall amount of THC produced, and on the ratio of THC to CBD.[16] Although overall cannabinoid production is influenced by environmental factors, the THC/CBD ratio is genetically determined and remains fixed throughout the life of a plant.[17] Non-drug plants produce relatively low levels of THC and high levels of CBD, while drug plants produce high levels of THC and low levels of CBD. When plants of these two chemotypes cross-pollinate, the plants in the first filial (F1) generation have an intermediate chemotype and produce similar amounts of CBD and THC. Female plants of this chemotype may produce enough THC to be utilized for drug production.[16][18]

Whether the drug and non-drug, cultivated and wild types of Cannabis constitute a single, highly variable species, or the genus is polytypic with more than one species, has been a subject of debate for well over two centuries. This is a contentious issue because there is no universally accepted definition of a species.[19] One widely applied criterion for species recognition is that species are "groups of actually or potentially interbreeding natural populations which are reproductively isolated from other such groups."[20] Populations that are physiologically capable of interbreeding, but morphologically or genetically divergent and isolated by geography or ecology, are sometimes considered to be separate species.[20] Physiological barriers to reproduction are not known to occur within Cannabis, and plants from widely divergent sources are interfertile.[11] However, physical barriers to gene exchange (such as the Himalayan mountain range) might have enabled Cannabis gene pools to diverge before the onset of human intervention, resulting in speciation.[21] It remains controversial whether sufficient morphological and genetic divergence occurs within the genus as a result of geographical or ecological isolation to justify recognition of more than one species.[22][23][24]
Early classifications
The Cannabis genus was first classified using the "modern" system of taxonomic nomenclature by Carolus Linnaeus in 1753, who devised the system still in use for the naming of species.[25] He considered the genus to be monotypic, having just a single species that he named Cannabis sativa L. (L. stands for Linnaeus, and indicates the authority who first named the species). Linnaeus was familiar with European hemp, which was widely cultivated at the time. In 1785, noted evolutionary biologist Jean-Baptiste de Lamarck published a description of a second species of Cannabis, which he named Cannabis indica Lam.[26] Lamarck based his description of the newly named species on plant specimens collected in India. He described C. indica as having poorer fiber quality than C. sativa, but greater utility as an inebriant. Additional Cannabis species were proposed in the 19th century, including strains from China and Vietnam (Indo-China) assigned the names Cannabis chinensis Delile, and Cannabis gigantea Delile ex Vilmorin.[27] However, many taxonomists found these putative species difficult to distinguish. In the early 20th century, the single-species concept was still widely accepted, except in the Soviet Union where Cannabis continued to be the subject of active taxonomic study. The name Cannabis indica was listed in various Pharmacopoeias, and was widely used to designate Cannabis suitable for the manufacture of medicinal preparations.[28]
20th Century
In 1924, Russian botanist D.E. Janichevsky concluded that ruderal Cannabis in central Russia is either a variety of C. sativa or a separate species, and proposed C. sativa L. var. ruderalis Janisch. and Cannabis ruderalis Janisch. as alternative names.[15] In 1929, renown plant explorer Nikolai Vavilov assigned wild or feral populations of Cannabis in Afghanistan to C. indica Lam. var. kafiristanica Vav., and ruderal populations in Europe to C. sativa L. var. spontanea Vav.[18][27] In 1940, Russian botanists Serebriakova and Sizov proposed a complex classification in which they also recognized C. sativa and C. indica as separate species. Within C. sativa they recognized two subspecies: C. sativa L. subsp. culta Serebr. (consisting of cultivated plants), and C. sativa L. subsp. spontanea (Vav.) Serebr. (consisting of wild or feral plants). Serebriakova and Sizov split the two C. sativa subspecies into 13 varieties, including four distinct groups within subspecies culta. However, they did not divide C. indica into subspecies or varieties.[15][29] This excessive splitting of C. sativa proved too unwieldy, and never gained many adherents.

In the 1970s, the taxonomic classification of Cannabis took on added significance in North America. Laws prohibiting Cannabis in the United States and Canada specifically named products of C. sativa as prohibited materials. Enterprising attorneys for the defense in a few drug busts argued that the seized Cannabis material may not have been C. sativa, and was therefore not prohibited by law. Attorneys on both sides recruited botanists to provide expert testimony. Among those testifying for the prosecution was Dr. Ernest Small, while Dr. Richard E. Schultes and others testified for the defense. The botanists engaged in heated debate (outside of court), and both camps impugned the other's integrity.[22][23] The defense attorneys were not often successful in winning their case, because the intent of the law was clear.[30]
In 1976, Canadian botanist Ernest Small[31] and American taxonomist Arthur Cronquist published a taxonomic revision that recognizes a single species of Cannabis with two subspecies: C. sativa L. subsp. sativa, and C. sativa L. subsp. indica (Lam.) Small & Cronq.[27] The authors hypothesized that the two subspecies diverged primarily as a result of human selection; C. sativa subsp. sativa was presumably selected for traits that enhance fiber or seed production, whereas C. sativa subsp. indica was primarily selected for drug production. Within these two subspecies, Small and Cronquist described C. sativa L. subsp. sativa var. spontanea Vav. as a wild or escaped variety of low-intoxicant Cannabis, and C. sativa subsp. indica var. kafiristanica (Vav.) Small & Cronq. as a wild or escaped variety of the high-intoxicant type. This classification was based on several factors including interfertility, chromosome uniformity, chemotype, and numerical analysis of phenotypic characters.[16][27][32]
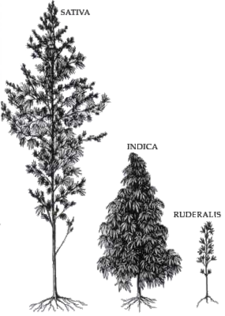
Professors William Emboden, Loran Anderson, and Harvard botanist Richard E. Schultes and coworkers also conducted taxonomic studies of Cannabis in the 1970s, and concluded that stable morphological differences exist that support recognition of at least three species, C. sativa, C. indica, and C. ruderalis.[33][34][35][36] For Schultes, this was a reversal of his previous interpretation that Cannabis is monotypic, with only a single species.[37] According to Schultes' and Anderson's descriptions, C. sativa is tall and laxly branched with relatively narrow leaflets, C. indica is shorter, conical in shape, and has relatively wide leaflets, and C. ruderalis is short, branchless, and grows wild in central Asia. This taxonomic interpretation was embraced by Cannabis aficionados who commonly distinguish narrow-leafed "sativa" drug strains from wide-leafed "indica" drug strains.[38]
Ongoing research
Molecular analytical techniques developed in the late twentieth century are being applied to questions of taxonomic classification. This has resulted in many reclassifications based on evolutionary systematics. Several studies of Random Amplified Polymorphic DNA (RAPD) and other types of genetic markers have been conducted on drug and fiber strains of Cannabis, primarily for plant breeding and forensic purposes.[39][40][41][42][43] Dutch Cannabis researcher E.P.M. de Meijer and coworkers described some of their RAPD studies as showing an "extremely high" degree of genetic polymorphism between and within populations, suggesting a high degree of potential variation for selection, even in heavily selected hemp cultivars.[17] They also commented that these analyses confirm the continuity of the Cannabis gene pool throughout the studied accessions, and provide further confirmation that the genus comprises a single species.
Karl W. Hillig, a graduate student in the laboratory of long-time Cannabis researcher Paul G. Mahlberg[44] at Indiana University, conducted a systematic investigation of genetic, morphological, and chemotaxonomic variation among 157 Cannabis accessions of known geographic origin, including fiber, drug, and feral populations. In 2004, Hillig and Mahlberg published a chemotaxomic analysis of cannabinoid variation in their Cannabis germplasm collection. They used gas chromatography to determine cannabinoid content and to infer allele frequencies of the gene that controls CBD and THC production, within the studied populations.[18] Hillig and Mahlberg concluded that the patterns of cannabinoid variation support recognition of C. sativa and C. indica as separate species, but not C. ruderalis. The authors assigned fiber/seed landraces and feral populations from Europe, central Asia, and Asia Minor to C. sativa. Narrow-leaflet and wide-leaflet drug accessions, southern and eastern Asian hemp accessions, and feral Himalayan populations were assigned to C. indica. In 2005, Hillig published a genetic analysis of the same set of accessions (this paper was submitted ahead of his 2004 manuscript with Mahlberg, but was delayed in publication), and proposed a three-species classification, recognizing C. sativa, C. indica, and (tentatively) C. ruderalis.[21] In his doctoral dissertation published the same year, Hillig stated that principal components analysis of phenotypic (morphological) traits failed to differentiate the putative species, but that canonical variates analysis resulted in a high degree of discrimination of the putative species and infraspecific taxa.[45] Another paper published by Hillig on chemotaxonomic variation in the terpenoid content of the essential oil of Cannabis revealed that several wide-leaflet drug strains in their collection had relatively high levels of certain sesquiterpene alcohols, including guaiol and isomers of eudesmol, that set them apart from the other putative taxa.[46] Hillig concluded that the patterns of genetic, morphological, and chemotaxonomic variation support recognition of C. sativa and C. indica as separate species. He also concluded there is little support to treat C. ruderalis as a separate species from C. sativa at this time, but more research on wild and weedy populations is needed because they were underrepresented in their collection.
As of 2007, most taxonomy web sites continue to list Cannabis as a single species.[47][48][49][50]
Popular usage
The scientific debate regarding taxonomy has had little effect on the terminology in widespread use among cultivators and users of drug-type Cannabis. Cannabis aficionados recognize three distinct types based on such factors as morphology, native range, aroma, and subjective psychoactive characteristics. "Sativa" is the term used to describe the most widespread variety, which is usually tall, laxly branched, and found in warm lowland regions. "Indica" is used to designate shorter, bushier plants adapted to cooler climates and highland environments. "Ruderalis" is the term used to describe the short plants that grow wild in Europe and central Asia.
Breeders, seed companies, and cultivators of drug type Cannabis often describe the ancestry or gross phenotypic characteristics of cultivars by categorizing them as "pure indica," "mostly indica," "indica/sativa," "mostly sativa", or "pure sativa."
In September 2005, New Scientist reported that researchers at the Canberra Institute of Technology had identified a new type of Cannabis based on analysis of mitochondrial and chloroplast DNA.[51] The New Scientist story, which was picked up by many news agencies and web sites, indicated that the research was to be published in the journal Forensic Science International. As of 25 Feb 2007 the article is listed as "in press," and there is no mention in the abstract of "Rasta."[52]
Wild cannabis
Wild C. sativa subsp. indica is mainly confined to hashish producing areas such as Afghanistan, and parts of Morocco. In the U.S. wild cannabis can grow wild in mid-west areas such as Kansas and Nebraska. This type is not valued for recreational use and is viewed as a weed by farmers. Wild C. sativa subsp. sativa shows great local variation; for example, in warm places, it can reach heights up to 20 feet (6 m) tall, but in colder climates it can be as short as 1 foot (30 cm) in height. Almost every single flower branch bears a seed. The wild C. sativa subsp. sativa has long, thin and airy buds and a Christmas tree shape structure. Wild C. sativa subsp. indica remains compact and bushy with thick buds for the most part, and is sometimes used by the locals for hashish production. Generally, there are far fewer seeds in wild C. sativa subsp. indica.
In many areas, wild or naturalized populations of Cannabis are considered invasive species, and are often targeted by government-sponsored eradication programmes.
Reproduction
Breeding systems


Cannabis is predominantly dioecious,[9][53] although many monoecious varieties have been described.[54] Subdioecy (the occurrence of monoecious individuals and dioecious individuals within the same population) is widespread.[55][56][57] Many populations have been described as sexually labile.[58][59][41]
As a result of intensive selection in cultivation, Cannabis exhibits many sexual phenotypes that can be described in terms of the ratio of female to male flowers occurring in the individual, or typical in the cultivar.[60] Dioecious varieties are preferred for drug production, where the female plants are preferred. Dioecious varieties are also preferred for textile fiber production, whereas monoecious varieties are preferred for pulp and paper production. It has been suggested that the presence of monoecy can be used to differentiate between licit crops of monoecious hemp and illicit dioecious drug crops.[55]
Mechanisms of sex determination
Cannabis has been described as having one of the most complicated mechanisms of sex determination among the dioecious plants.[60] Many models have been proposed to explain sex determination in Cannabis.
Based on studies of sex reversal in hemp, it was first reported by K. Hirata in 1924 that an XY sex-determination system is present.[58] At the time, the XY system was the only known system of sex determination. The X:A system was first described in Drosophila spp in 1925.[61] Soon thereafter, Schaffner disputed Hirata's interpretation,[62] and published results from his own studies of sex reversal in hemp, concluding that an X:A system was in use and that furthermore sex was strongly influenced by environmental conditions.[59]
Since then, many different types of sex determination systems have been discovered, particularly in plants.[53] Dioecy is relatively uncommon in the plant kingdom, and a very low percentage of dioecious plant species have been determined to use the XY system. In most cases where the XY system is found it is believed to have evolved recently and independently.[63]
Since the 1920s, a number of sex determination models have been proposed for Cannabis. Ainsworth describes sex determination in the genus as using "an X/autosome dosage type".[53]

The question of whether heteromorphic sex chromosomes are indeed present is most conveniently answered if such chromosomes were clearly visible in a karyotype. Cannabis was one of the first plant species to be karyotyped; however, this was in a period when karyotype preparation was primitive by modern standards (see History of Cytogenetics). Heteromorphic sex chromosomes were reported to occur in staminate individuals of dioecious "Kentucky" hemp, but were not found in pistillate individuals of the same variety. Dioecious "Kentucky" hemp was assumed to use an XY mechanism. Heterosomes were not observed in analyzed individuals of monoecious "Kentucky" hemp, nor in an unidentified German cultivar. These varieties were assumed to have sex chromosome composition XX.[64] According to other researchers, no modern karyotype of Cannabis had been published as of 1996.[65] Proponents of the XY system state that Y chromosome is slightly larger than the X, but difficult to differentiate cytologically.[66]
More recently, Sakamoto and various co-authors[67][68] have used RAPD to isolate several genetic marker sequences that they name Male-Associated DNA in Cannabis (MADC), and which they interpret as indirect evidence of a male chromosome. Several other research groups have reported identification of male-associated markers using RAPD and AFLP.[69][41][17] Ainsworth commented on these findings, stating,
It is not surprising that male-associated markers are relatively abundant. In dioecious plants where sex chromosomes have not been identified, markers for maleness indicate either the presence of sex chromosomes which have not been distinguished by cytological methods or that the marker is tightly linked to a gene involved in sex determination.[53]
Environmental sex determination is known to occur in a variety of species.[70] Many researchers have suggested that sex in Cannabis is determined or strongly influenced by environmental factors.[59] Ainsworth reviews that treatment with auxin and ethylene have feminizing effects, and that treatment with cytokinins and gibberellins have masculinizing effects.[53] It has been reported that sex can be reversed in Cannabis using chemical treatment.[71] A PCR-based method for the detection of female-associated DNA polymorphisms by genotyping has been developed.[72]
Various strains of cannabis
Although there are hundreds of strains of cannabis in existence, there are also many rumors and urban legends. Many alleged strains, such as Purple Haze, are very predominant in pop-culture (see right), but the actual existence of many of these strains is uncertain and the slang terms used to refer to these strains do not appear to be used by botanists. Some strains, such as G-13, are acknowledged to be urban legends.[73]
Strains of cannabis:
- Acapulco gold
- BC Bud
- Cinderella 99
- Chocolate Thai
- Panama Red
- G-13
- Kush
- Northern Lights
- Purple Haze
- Quebec Gold
- White Widow
Some of the strains' names, such as Chocolate Thai,[74] popular in the early 1990s due to its supposed high potency,[75] entered the mass culture. For example, Chocolate Thai was adopted as a stage name of a jazz performer, whose album The Real McCoy was released in 2006.[76] It should be noted, however, that because there is no manufacturing or state control over the process of production of cannabis, many "strains" may in fact be just marketing brands adopted by drug dealers to increase sales.
Aspects of Cannabis production and use

- Medical Cannabis discusses its use as a medication.
- Cannabis (drug) discusses its use as a recreational drug.
- Spiritual use of cannabis discusses sacramental and religious use.
- Hemp discusses its uses as a source of housing, oil, food, fibers, and industrial materials.
- Cannabis (drug) cultivation discusses aspects of cultivation for medicinal and recreational drug purposes
- Legality of cannabis focuses on the law and enforcement aspects of growing, transporting, selling and using cannabis as a drug.
- Cannabis rescheduling in the United States
- Drug policy of the Netherlands
- Health issues and the effects of cannabis discusses the pharmacology, physical, and mental effects of Cannabis when used as drug.
Gallery of images
See also
- Medical Cannabis
- Cannabis (drug)
- Legality of cannabis by country
References
- ↑ 1.0 1.1 1.2 1.3 "Cannabis sativa information from NPGS/GRIN". www.ars-grin.gov. Retrieved on 2008-07-13.
- ↑ 2.0 2.1 Erowid. 2006. Cannabis Basics. Retrieved on 25 February 2007
- ↑ Rubin, Vera, ed. (1976), written at The Hague, Cannabis and Culture, Mouton, pp. 305, ISBN 9027976694
- ↑ Black, Jeremy; George, Andrew; Nicholas, Postgate, eds. (1999), A Concise Dictionary of Akkadian, SANTAG, 5, Wiesbaden: Harrassowitz Verlag, ISBN 3-447-04225-7
- ↑ Lebel-Hardenack, S. and S. R. Grant. 1997. Genetics of sex determination in flowering plants. Trends in Plant Science 2(4): 130–136.
- ↑ Cristiana Moliterni, V. M., L. Cattivelli, P. Ranalli. and G. Mandolino. 2005. The sexual differentiation of Cannabis sativa L.: A morphological and molecular study. Euphytica 140(1-2): 95-106. Retrieved on 25 February 2007
- ↑ Bouquet, R. J. 1950. Cannabis. United Nations Office on Drugs and Crime. Retrieved on 23 February 2007
- ↑ Mahlberg, Paul G. and Eun Soo Kim. 2001. THC (tetrahyrdocannabinol) accumulation in glands of Cannabis (Cannabaceae). The Hemp Report 3(17). Retrieved on 23 February 2007
- ↑ 9.0 9.1 9.2 Clarke, Robert C. 1991. Marijuana Botany, 2nd ed. Ron Publishing, California. ISBN 0-914171-78-X
- ↑ Small, E. 1975. Morphological variation of achenes of Cannabis. Canadian Journal of Botany 53(10): 978-987.
- ↑ 11.0 11.1 Small, E. 1972. Interfertility and chromosomal uniformity in Cannabis. Canadian Journal of Botany 50(9): 1947-1949.
- ↑ Schultes, R. E., A. Hofmann, and C. Rätsch. 2001. The nectar of delight. In: Plants of the Gods 2nd ed., Healing Arts Press, Rochester, Vermont, pp. 92-101. ISBN 0-89281-979-0
- ↑ Song, B.-H., Wang, X.-Q., Li, F.-Z., and Hong, D.-Y. 2001. Further evidence for paraphyly of the Celtidaceae from the chloroplast gene matK. Plant Systematics and Evolution 228(1-2): 107-115.
- ↑ Sytsma, K. J., Morawetz, J., Pires, J. C., Nepokroeff, M., Conti, E., Zjhra, M., Hall, J. C., and Chase, M. W. 2002. Urticalean Rosids: circumscription, Rosid ancestry, and phylogenetics based on rbcL, trnL-F, and ndh-F sequences. American Journal of Botany 89(9): 1531-1546.
- ↑ 15.0 15.1 15.2 Small, Ernest. 1975. American law and the species problem in Cannabis: Science and semantics. Bulletin on Narcotics 27(3): 1-20. Retrieved on 23 February 2007
- ↑ 16.0 16.1 16.2 Small, E. and H. D. Beckstead. 1973. Common cannabinoid phenotypes in 350 stocks of Cannabis. Lloydia 36: 144–165.
- ↑ 17.0 17.1 17.2 Etienne P. M. de Meijer, M. Bagatta, A. Carboni, P. Crucitti, V. M. Cristiana Moliterni, P. Ranalli, and G. Mandolino. 2003. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 163(1): 335-346. Retrieved on 23 February 2007
- ↑ 18.0 18.1 18.2 Hillig, Karl W. and Paul G. Mahlberg. 2004. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). American Journal of Botany 91(6): 966-975. Retrieved on 22 February 2007
- ↑ Small, E. 1979. Fundamental aspects of the species problem in biology. In: The Species Problem in Cannabis, vol. 1: Science. Corpus Information Services, Toronto, Canada, pp. 5-63. ISBN 0-919217-11-7
- ↑ 20.0 20.1 Rieger, R., A. Michaelis, and M. M. Green. 1991. Glossary of Genetics, 5th ed. Springer-Verlag, pp. 458-459. ISBN 0-387-52054-6
- ↑ 21.0 21.1 Hillig, Karl W. 2005. Genetic evidence for speciation in Cannabis (Cannabaceae). Genetic Resources and Crop Evolution 52(2): 161-180. Retrieved on 23 February 2007
- ↑ 22.0 22.1 Small, E. 1975. On toadstool soup and legal species of marihuana. Plant Science Bulletin 21(3): 34-39. Retrieved on 23 February 2007
- ↑ 23.0 23.1 Emboden, W. A. 1981. The genus Cannabis and the correct use of taxonomic categories. Journal of Psychoactive Drugs 13: 15–21.
- ↑ Schultes, R. E., and A. Hofmann. 1980. Botany and Chemistry of Hallucinogens. C. C. Thomas, Springfield, Illinois, pp. 82–116. ISBN 0-398-03863-5
- ↑ Linnaeus, C. 1753. Species Plantarum 2: 1027. Salvius, Stockholm. [Facsimile edition, 1957-1959. Ray Society, London, U.K.]
- ↑ de Lamarck, J.B. 1785. Encyclopédie Méthodique de Botanique, vol. 1, pt. 2. Paris, France, pp. 694-695
- ↑ 27.0 27.1 27.2 27.3 Small, E. and A. Cronquist. 1976. A practical and natural taxonomy for Cannabis. Taxon 25(4): 405–435.
- ↑ Winek, C. L. 1977. Some historical aspects of marijuana. Clinical Toxicology 10(2): 243-253.
- ↑ Serebriakova T. Ya. and I. A. Sizov. 1940. Cannabinaceae Lindl. In: Vavilov N. I. (ed.), Kulturnaya Flora SSSR, vol. 5, Moscow-Leningrad, USSR, pp. 1-53. [in Russian]
- ↑ Watts, G. 2006. Cannabis confusions. BMJ 332: 175-176. Retrieved on 23 February 2007
- ↑ Ernest Small (biography). National Research Council Canada. Retrieved on 23 February 2007
- ↑ Small, E., P. Y. Jui, and L. P. Lefkovitch. 1976. A numerical taxonomic analysis of Cannabis with special reference to species delimitation. Systematic Botany 1(1): 67-84.
- ↑ Schultes, R. E., W. M. Klein, T. Plowman, and T. E. Lockwood. 1974. Cannabis: an example of taxonomic neglect. Harvard University Botanical Museum Leaflets 23: 337–367.
- ↑ Anderson, L. C. 1974. A study of systematic wood anatomy in Cannabis. Harvard University Botanical Museum Leaflets 24: 29–36. Retrieved on 23 February 2007
- ↑ Anderson, L. C. 1980. Leaf variation among Cannabis species from a controlled garden. Harvard University Botanical Museum Leaflets 28: 61–69. Retrieved on 23 February 2007
- ↑ Emboden, W. A. 1974. Cannabis – a polytypic genus. Economic Botany 28: 304-310.
- ↑ Schultes, R. E. 1970. Random thoughts and queries on the botany of Cannabis. In: Joyce, C. R. B. and Curry, S. H. (eds), The Botany and Chemistry of Cannabis. J. & A. Churchill, London, pp. 11-38.
- ↑ Interview with Robert Connell Clarke. 1 Jan 2005. NORML, New Zealand. Retrieved on 19 February 2007
- ↑ Faeti, V., G. Mandolino, and P. Ranalli. 1996. Genetic diversity of Cannabis sativa germplasm based on RAPD markers. Plant Breeding 115: 367–370.
- ↑ Forapani, S., A. Carboni, C. Paoletti, V. M. Christiana Moliterni, P. Ranalli, and G. Mandolino. 2001. Comparison of hemp (Cannabis sativa L.) varieties using Random Amplified Polymorphic DNA markers. Crop Science 41: 1682-1689. Retrieved on 23 February 2007
- ↑ 41.0 41.1 41.2 Mandolino, G. and Ranalli, P. 2002. The applications of molecular markers in genetics and breeding of hemp. Journal of Industrial Hemp 7(1): 7-23. Retrieved on 23 February 2007
- ↑ Gilmore S., R. Peakall, and J. Roberts. 2003. Short tandem repeats (STR) DNA markers are hypervariable and informative in Cannabis sativa: implications for forensic investigations. Forensic Science International 131(1): 65-74. Retrieved on 25 February 2007
- ↑ Kojoka M., O. Iida, Y. Makino, S. Sekita, and M. Satake. 2002. DNA fingerprinting of Cannabis sativa using inter-simple sequence repeat (ISSR) amplification. Planta Medica 68(1): 60-63.
- ↑ Dr. Paul G. Mahlberg's Cannabis Research. North American Industrial Hemp Council. Retrieved on 23 February 2007
- ↑ Hillig, Karl William. 2005. A systematic investigation of Cannabis. Doctoral Dissertation. Department of Biology, Indiana University. Bloomington, Indiana. Published by UMI. Retrieved on 23 February 2007
- ↑ Hillig, Karl W. 2004. A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochemical Systematics and Ecology 32: 875-891. Retrieved on 23 February 2007
- ↑ USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN), National Germplasm Resources Laboratory, Beltsville, Maryland. Retrieved on 23 February 2007
- ↑ Barlow, Snow. 2006. Sorting Cannabis names. Multilingual Multiscript Plant Name Database. The University of Melbourne. Retrieved on 23 February 2007
- ↑ Integrated Taxonomic Information System (ITIS). Retrieved on 23 February 2007
- ↑ The Taxonomicon. Universal Taxonomic Services. Retrieved on 23 February 2007
- ↑ 2005. Rasta lends its name to a third type of Cannabis. New Scientist 2517: 12. Retrieved on 24 February 2007
- ↑ Gilmore, S., R. Peakall, and J. Robertson. 2007. Organelle DNA haplotypes reflect crop-use characteristics and geographic origins of Cannabis sativa. Forensic Science International. In Press. Retrieved on 25 February 2007
- ↑ 53.0 53.1 53.2 53.3 53.4 Ainsworth, C. 2000. Boys and girls come out to play: the molecular biology of dioecious plants. Annals of Botany 86(2): 211-221. Retrieved on 24 February 2007
- ↑ de Meijer, E. P. M. 1999. Cannabis germplasm resources. In: Ranalli P. (ed.). Advances in Hemp Research, Haworth Press, Binghamton, NY, pp. 131-151. ISBN 1-56022-872-5
- ↑ 55.0 55.1 "Cannabis as a licit crop: recent developments in Europe".
- ↑ Schumann, E., A. Peil, and W. E. Weber. 1999. Preliminary results of a German field trial with different hemp (Cannabis sativa L.) accessions. Genetic Resources and Crop Evolution 46(4): 399-407. Retrieved on 24 February 2007
- ↑ Ranalli, P. 2004. Current status and future scenarios of hemp breeding. Euphytica 140(1): 121-131.
- ↑ 58.0 58.1 Hirata, K. 1924. Sex reversal in hemp. Journal of the Society of Agriculture and Forestry 16: 145-168.
- ↑ 59.0 59.1 59.2 Schaffner, J. H. 1931. The fluctuation curve of sex reversal in staminate hemp plants induced by photoperiodicity. American Journal of Botany 18(6): 424-430.
- ↑ 60.0 60.1 Truta, E., E. Gille, E. Toth, and M. Maniu. 2002. Biochemical differences in Cannabis sativa L. depending on sexual phenotype. Journal of Applied Genetics 43(4): 451-462. Retrieved on 24 February 2007
- ↑ Bridges, C. B. 1925. Sex in relation to chromosomes and genes. American Naturalist 59: 127-137.
- ↑ Schaffner, J. H. 1929. Heredity and sex. Ohio Journal of Science 29(1): 289-300.
- ↑ Negrutiu, I., B. Vyskot, N. Barbacar, S. Georgiev, and F. Moneger. 2001. Dioecious plants; a key to the early events of sex chromosome evolution. Plant Physiology 127(4): 418-424.
- ↑ Menzel, Margaret Y. 1964. Meiotic chromosomes of monoecious Kentucky hemp (Cannabis sativa). Bulletin of the Torrey Botanical Club 91(3): 193-205.
- ↑ Shao Hong and Robert C. Clarke. 1996. Taxonomic studies of Cannabis in China. Journal of the International Hemp Association 3(2): 55-60. Retrieved on 25 February 2007
- ↑ Peil, A., H. Flachowsky, E. Schumann, and W. E. Weber. 2003. Sex-linked AFLP markers indicate a pseudoautosomal region in hemp (Cannabis sativa L.). Theoretical and Applied Genetics 107(1): 102-109.
- ↑ Sakamoto, K., K. Shimomura, Y. Komeda, H. Kamada, and S. Satoh. 1995. A male-associated DNA sequence in a dioecious plant, Cannabis sativa L. Plant & Cell Physiology 36(8): 1549-1554. Retrieved on 25 February 2007
- ↑ Sakamoto, K., T. Abe, T. Matsuyama, S. Yoshida, N. Ohmido, K. Fukui, and S. Satoh. 2005. RAPD markers encoding retrotransposable elements are linked to the male sex in Cannabis sativa L. Genome 48(5): 931-936. Retrieved on 25 February 2007
- ↑ Törjék, O., N. Bucherna, E. Kiss, H. Homoki, Z. Finta-Korpelová, I. Bócsa, I. Nagy, and L. E. Heszky. 2002. Novel male specific molecular markers (MADC5, MADC6) for sex identification in hemp. Euphytica 127: 209-218.
- ↑ Tanurdzic, M. and J. A. Banks. 2004. Sex-determining mechanisms in land plants. Plant Cell 16 (suppl.): S61-71.
- ↑ Mohan Ram, H. Y., and R. Sett. 1982. Induction of fertile male flowers in genetically female Cannabis sativa plants by silver nitrate and silver thiosulfate anionic complex. Theoretical and Applied Genetics 62: 369-375.
- ↑ Journal of Industrial Hemp 2003 Vol 8 issue 1 page 5-9, Female-Associated DNA Polymorphisms of Hemp (Cannabis sativa L.), Hong Shao, Shu-Juan Song, Robert C. Clarke
- ↑ Doorenbos, Norman J., Patricia S. Fetterman, Maynard W. Quimby, and Carlton Turner. 1971. Cultivation, extraction, and analysis of Cannabis sativa L. Annals New York Academy of Sciences 191: 3-14.
- ↑ Hirsch, Robert; Edited by Judith Pynchon (1997). "Clinicians' self-assessment questions and answers in substance abuse treatment". Journal of Substance Abuse Treatment (Journal of Substance Abuse) 14 (1): 95–98. doi:.
- ↑ "Strains of Yesteryear" by DJ Short
- ↑ Answers.com album list
Further reading
- Cannabis: A History (2005) Martin Booth ISBN 0-312-32220-8
- UNODC: World Drug Report 2006, Chapter 2: Cannabis: Why We Should Care (2006)
- EMCDDA drugs profile: Cannabis (2007)
- Herer, Jack. The Emperor Wears No Clothes: The Authoritative Historical Record of Cannabis and the Conspiracy Against Marijuana. Ah Ha Publishing Company, 2000. ISBN 1-878125-02-8
- Herer, Jack. Internet edition of The Emperor Wears No Clothes
External links
- International Plant Names Index (IPNI)
- The Endocannabinoid System Network (ECSN) - Contains medical information to the Endocannabinoid System
- Erowid: Cannabis (Marijuana) Vault.
|
||||||||||||||||||||
|
|||||||||||
|
|||||
|
||||||||||||||||||||||||||||||||||||||||||