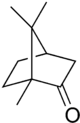
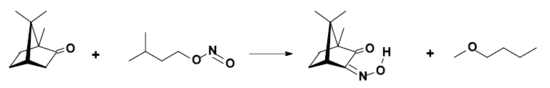Camphor
| Camphor[1][2] | |
|---|---|
 |
|
| IUPAC name | 1,7,7-trimethylbicyclo [2.2.1]heptan-2-one |
| Other names | 2-bornanone, 2-camphanone bornan-2-one, Formosa |
| Identifiers | |
| CAS number | [76-22-2] (unspecified) [464-49-3] ((1R)-Camphor) [464-48-2] ((1S)-Camphor} |
| RTECS number | EX1260000 (R) EX1250000 (S) |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C10H16O |
| Molar mass | 152.23 |
| Appearance | White or colorless crystals |
| Density | 0.990 (solid) |
| Melting point |
179.75 °C (452.9 K) |
| Boiling point |
204 °C (477 K) |
| Solubility in water | 0.12 g in 100 ml |
| Solubility in chloroform | ~100 g in 100 ml |
| Chiral rotation [α]D | +44.1° |
| Hazards | |
| Main hazards | flammable |
| NFPA 704 |
 2
2
0
|
| R-phrases | 11-20/21/22-36/37/38 |
| S-phrases | 16-26-36 |
| Related compounds | |
| Related ketone | fenchone,thujone |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Camphor is a waxy, white or transparent solid with a strong, aromatic odor.[3] It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel (Cinnamomum camphora), a large evergreen tree found in Asia (particularly in Borneo and Taiwan, hence its alternate name). It also occurs in some other related trees in the laurel family, notably Ocotea usambarensis. It can also be synthetically produced from oil of turpentine. It is used for its scent, as an ingredient in cooking (mainly in India), as an embalming fluid, in religious ceremonies and for medicinal purposes. A major source of camphor in Asia is camphor basil.
Contents |
History
The word camphor derives from the French word camphre, itself from Medieval Latin camfora, from Arabic kafur, from Malay kapur Barus.[4] Barus was the port on the western coast of the Indonesian island of Sumatra where foreign traders would call to buy camphor. In the Indian language Sanskrit, the word for camphor is karpoor. A South-Indian adaptation of this word, karpooram, is found in many South-Indian/Dravidian languages (like Telugu, Tamil, Kannada and Malayalam).
In the 9th century, the Arab chemist, Al-Kindi (known as Alkindus in Europe), provided the earliest recipe for the production of camphor in his Kitab Kimiya' al-'Itr (Book of the Chemistry of Perfume).[5]
Already in the 19th century, it was known that with nitric acid, camphor could be oxidized into camphoric acid. Haller and Blanc published a semisynthesis of camphor from camphoric acid, which, although demonstrating its structure, would not prove it. The first complete total synthesis for camphoric acid was published by Gustaf Komppa in 1903. Its starting materials were diethyl oxalate and 3,3-dimethylpentanoic acid, which reacted by Claisen condensation to give diketocamphoric acid. Methylation with methyl iodide and a complicated reduction procedure produced camphoric acid. William Perkin published another synthesis a short time later. Previously, some organic compounds (such as urea) had been synthesized in the laboratory as a proof of concept, but camphor was a scarce natural product with a worldwide demand. Komppa realized this and began industrial production of camphor in Tainionkoski, Finland, in 1907.
Norcamphor is a camphor derivative with the three methyl groups replaced by hydrogen.
Other substances deriving from trees are sometimes wrongly sold as camphor.
Uses
Modern uses include as a plasticizer for nitrocellulose, as a moth repellent, as an antimicrobial substance, in embalming, and in fireworks. Solid camphor releases fumes that form a rust-preventative coating and is therefore stored in tool chests to protect tools against rust.[6] Camphor crystals are also used to prevent damage to insect collections by other small insects.
It is also used in medicine. Camphor is readily absorbed through the skin and produces a feeling of cooling similar to that of menthol and acts as slight local anesthetic and antimicrobial substance. There are anti-itch gel and cooling gels with camphor as the active ingredient. Camphor is an active ingredient (along with menthol) in vapor-steam products, such as Vicks VapoRub, and it is effective as a cough suppressant. It may also be administered orally in small quantities (50 mg) for minor heart symptoms and fatigue.[7]
In the 18th Century, it was used by Auenbrugger in the treatment of mania.
It is also believed that camphor will deter snakes and other reptiles due to its strong odor. Similarly, camphor is believed to be toxic to insects and is thus sometimes used as a repellent.
Camphor is widely used in Hindu religious ceremonies. Hindus worship by lighting a holy flame by burning camphor and forms the most important part of most religious ceremonies. Camphor is used in the Mahashivratri celebrations of Shiva, the Hindu god of destruction of (re)creation. As a natural pitch substance it burns cool without leaving an ash residue, which symbolizes consciousness. Of late, most Temples in Dravidian land have stopped lighting camphor in the Main Sanctum Sanctorium due to heavy deposits of carbon, however, open areas do use camphor.
It is also found in clarifying masks used for skin.
Recently, carbon nanotubes were successfully synthesized using camphor in chemical vapor deposition process.[8]
Culinary
Currently, camphor is mostly used as a flavoring for sweets in Asia. In ancient and medieval Europe it was widely used as ingredient for sweets but it is now mainly used for medicinal purposes. Camphor was used as a flavoring in confections resembling ice cream in China during the Tang dynasty (A.D. 618-907). Camphor is widely used in cooking (mainly for dessert dishes) in India where it is known as Pachha Karpooram (literally meaning "green camphor" though "Pachha" in Tamil can also be translated to mean "raw" which is "Pachha Karpooram's" intended meaning). It is widely available at Indian grocery stores and is labeled as "Edible Camphor." In Hindu pujas and ceremonies, camphor is burned in a ceremonial spoon for performing aarti. This type of camphor is also sold at Indian grocery stores but it is not suitable for cooking. The only type that should be used for food are those which are labeled as "Edible Camphor."
Toxicology
In larger quantities, it is poisonous when ingested and can cause seizures, confusion, irritability, and neuromuscular hyperactivity. Despite the rather low skin absorption it may still lead to hepatotoxicity in the extreme cases.[9] [10] Lethal doses in adults are in the range 50–500 mg/kg (orally). Generally, 2 g causes serious toxicity and 4 g is potentially lethal.
In 1980, the United States Food and Drug Administration set a limit of 11% allowable camphor in consumer products and totally banned products labeled as camphorated oil, camphor oil, camphor liniment, and camphorated liniment (except "white camphor essential oil" contains no significant amount of camphor). Since alternative treatments exist, medicinal use of camphor is discouraged by the FDA, except for skin-related uses, such as medicated powders, which contain only small amounts of camphor.
Reactions
Typical camphor reactions are:
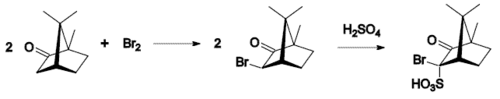
- bromination
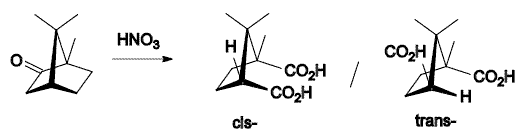
- oxidation with nitric acid
- conversion to isonitrosocamphor
Camphor can also be reduced to isoborneol using sodium borohydride.
Biosynthesis
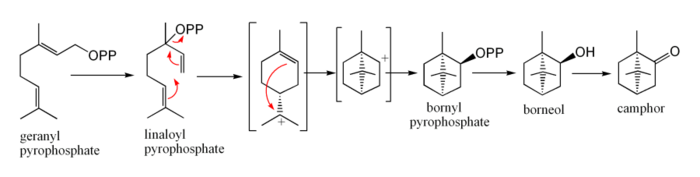
In biosynthesis camphor is produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate to bornyl pyrophosphate, followed by hydrolysis to borneol and oxidation to camphor.
References
- ↑ The Merck Index, 7th edition, Merk & Co, Rahway, New Jersey, USA, 1960
- ↑ Handbook of Chemistry and Physics, CRC Press, Ann Arbor, Michigan
- ↑ Mann JC, Hobbs JB, Banthorpe DV, Harborne JB (1994). Natural products: their chemistry and biological significance. Harlow, Essex, England: Longman Scientific & Technical. pp. 309–11. ISBN 0-582-06009-5.
- ↑ http://www.etymonline.com/index.php?search=camphor
- ↑ Al-Kindi, FSTC
- ↑ Tips for Cabinet Making Shops
- ↑ National Agency for Medicines
- ↑ Kumar M, Ando Y (2007). "Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology". J Phys Conf Ser. 61: 643–6. doi:. http://www.iop.org/EJ/abstract/1742-6596/61/1/129.
- ↑ Martin D, Valdez J, Boren J, Mayersohn M (Oct 2004). "Dermal absorption of camphor, menthol, and methyl salicylate in humans". J Clin Pharmacol 44 (10): 1151–7. doi:. PMID 15342616.
- ↑ Uc A, Bishop WP, Sanders KD (Jun 2000). "Camphor hepatotoxicity". South Med J. 93 (6): 596–8. PMID 10881777. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0038-4348&volume=93&issue=6&spage=596.
External links
- Extensive Camphor Info (www.siu.edu)
- Camphor Evidence-based Monograph from Natural Medicines Comprehensive Database