Butyric acid
| Butyric acid | |
|---|---|
 |
|
 |
|
 |
|
| IUPAC name | Butanoic acid |
| Identifiers | |
| CAS number | 107-92-6 |
| PubChem | |
| MeSH | |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C4H8O2 |
| Molar mass | 88.1051 g/mol |
| Density | 0.96 g/mL |
| Melting point |
-7.9 °C (265.1 K) |
| Boiling point |
163.5 °C (436.5 K) |
| Solubility in water | miscible |
| Hazards | |
| Main hazards | Corrosive; Harmful to aquatic organisms |
| R-phrases | 34 |
| S-phrases | 26 36 45 |
| Flash point | 72 °C |
| Autoignition temperature |
452 °C |
| RTECS number | ES5425000 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
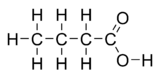
Butyric acid (from Greek βούτυρος = butter), also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. It is found in rancid butter, parmesan cheese, vomit, and body odor and has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). Butyric acid can be detected by mammals with good scent detection abilities such as dogs at 10 ppb, whereas humans can detect it in concentrations above 10 ppm.
Contents |
Chemistry
Butyric acid is a fatty acid occurring in the form of esters in animal fats and plant oils. The triglyceride of butyric acid makes up 3% to 4% of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis leading to the unpleasant odor. It is an important member of the fatty acid sub-group called short chain fatty acids. Butyric acid is a weak acid with a pKa of 4.82, similar to acetic acid which has pKa 4.76.[1] The similar strength of these acids results from their common -CH2COOH terminal structure.[2] Pure butyric acid is 10.9 molar.
The acid is an oily colorless liquid that is easily soluble in water, ethanol, and ether, and can be separated from an aqueous phase by saturation with saslts such as calcium chloride. Potassium dichromate and sulfuric acid oxidize it to carbon dioxide and acetic acid, while alkaline potassium permanganate oxidizes it to carbon dioxide. The calcium salt, Ca(C4H7O2)2·H2O, is less soluble in hot water than in cold.
Butyric acid has a structural isomer called isobutyric acid (2-methylpropanoic acid).
Production
It is industrially prepared by the fermentation of sugar or starch, brought about by the addition of putrefying cheese, with calcium carbonate added to neutralize the acids formed in the process. The butyric fermentation of starch is aided by the direct addition of Bacillus subtilis. Salts and esters of the acid are called butanoates.
Butyric acid or fermentation butyric acid is also found as a hexyl ester (hexyl butanoate) in the oil of Heracleum giganteum (a type of cow parsnip) and as an octyl ester (octyl butanoate) in parsnip (Pastinaca sativa); it has also been noticed in the fluids of the flesh and in perspiration.
Uses
Butyric acid is used in the preparation of various butanoate esters. Low-molecular-weight esters of butyric acid, such as methyl butanoate, have mostly pleasant aromas or tastes. As a consequence, they find use as food and perfume additives. They are also used in organic laboratory courses, to teach the Fischer esterification reaction.
Biological functionality
Butanoate fermentation
Butanoate is produced as end-product of a fermentation process solely performed by obligate anaerobic bacteria. Fermented Kombucha "tea" includes butyric acid as a result of the fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861. Examples of butanoate-producing species of bacteria:
- Clostridium acetobutylicum
- Clostridium butyricum
- Clostridium kluyveri
- Clostridium pasteurianum
- Fusobacterium nucleatum
- Butyrivibrio fibrisolvens
- Eubacterium limosum
The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is then oxidized into acetyl coenzyme A using a unique mechanism that involves an enzyme system called pyruvate-ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of elemental hydrogen (H2) are formed as wastes products from the cell. Then:
| Action | Responsible enzyme |
| Acetyl coenzyme A converts into acetoacetyl coenzyme A | acetyl-CoA-acetyl transferase |
| Acetoacetyl coenzyme A converts into β-hydroxybutyryl CoA | β-hydroxybutyryl-CoA dehydrogenase |
| β-hydroxybutyryl CoA converts into crotonyl CoA | crotonase |
| Crotonyl CoA converts into butyryl CoA (CH3CH2CH2C=O-CoA) | butyryl CoA dehydrogenase |
| A phosphate group replaces CoA to form butyryl phosphate | phosphobutyrylase |
| The phosphate group joins ADP to form ATP and butyrate | butyrate kinase |
ATP is produced, as can be seen, in the last step of the fermentation. Three molecules of ATP are produced for each glucose molecule, a relatively high yield. The balanced equation for this fermentation is:
C6H12O6 → C4H8O2 + 2CO2 + 2H2
Acetone and butanol fermentation
Several species form acetone and butanol in an alternative pathway, which starts as butyrate fermentation. Some of these species are:
- Clostridium acetobutylicum: the most prominent acetone and butanol producer, used also in industry
- Clostridium beijerinckii
- Clostridium tetanomorphum
- Clostridium aurantibutyricum
These bacteria begin with butanoate fermentation as described above, but, when the pH drops below 5, they switch into butanol and acetone production in order to prevent further lowering of the pH. Two molecules of butanol are formed for each molecule of acetone.
The change in the pathway occurs after acetoacetyl CoA formation. This intermediate then takes two possible pathways:
- Acetoacetyl CoA → acetoacetate → acetone, or
- Acetoacetyl CoA → butyryl CoA → butanal → butanol.
Butyric acid function/activity
Highly-fermentable fibers like oat bran, pectin, and guar are transformed by colonic bacteria into short chain fatty acids including butyrate.
Butanoate has diverse and, it seems, paradoxical effects on cellular proliferation, apoptosis and differentiation that may be either pro-neoplastic or anti-neoplastic, depending upon factors such as the level of exposure, availability of other metabolic substrate, and the intracellular milieu. Butanoate is thought by some to be protective against colon cancer. However, not all studies support a chemopreventive effect, and the lack of agreement (particularly between in vivo and in vitro studies) on butyrate and colon cancer has been termed the "butyrate paradox." There are many reasons for this discrepant effect, including differences between the in vitro and in vivo environments, the timing of butanoate administration, the amount administered, the source (usually dietary fiber) as a potential confounder, and an interaction with dietary fat. Together, the studies suggest that the chemopreventive benefits of butanoate depend in part on amount, time of exposure with respect to the tumorigenic process, and the type of fat in the diet.[3] Low carbohydrate diets like the Atkins diet are known to reduce the amount of butanoate produced in the colon.
Butyric acid has been associated with the ability to inhibit the function of histone deacetylase enzymes, thereby favouring an acetylated state of histones in the cell. Acetylated histones have a lower affinity for DNA than non-acetylated histones, due to the neutralisation of electrostatic charge interactions. In general, it is thought that transcription factors will be unable to access regions where histones are tightly associated with DNA (ie non-acetylated, e.g., heterochromatin). Therefore, it is thought that butyric acid enhances the transcriptional activity at promoters, which are typically silenced/downregulated due to histone deacetylase activity.
References
See also
- Indole-3-butyric acid
- Acids in wine
External links
- International Chemical Safety Card 1334
- 2004 review of the scientific evidence on butanoate/butyrate vs. colon cancer
This article incorporates information from the 1911 encyclopedia.
|
||||||||||||||