Biotin
- Vitamin H redirects here. In medical slang, "vitamin H" may also refer to haloperidol.[1] In gamer slang, Vitamin H may also refer to the Halo series.
| Biotin | |
|---|---|
 |
|
 |
|
| IUPAC name | 5-[(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanoic acid |
| Other names | Vitamin B7; Vitamin H |
| Identifiers | |
| CAS number | 58-85-5 |
| PubChem | |
| SMILES |
|
| InChI |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C10H16N2O3S |
| Molar mass | 244.31 g mol−1 |
| Solubility in water | Soluble |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
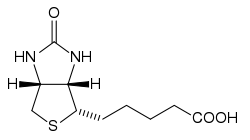
Biotin, also known as vitamin H or B7, has the chemical formula C10H16N2O3S (Biotin; Coenzyme R, Biopeiderm), is a water-soluble B-complex vitamin which is composed of an ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is a cofactor in the metabolism of fatty acids and leucine, and in gluconeogenesis.
Contents |
General overview
Biotin is necessary for cell growth, the production of fatty acids, and the metabolism of fats and amino acids. It plays a role in the Citric acid cycle, which is the process by which biochemical energy is generated during aerobic respiration. Biotin not only assists in various metabolic reactions, but also helps to transfer carbon dioxide. Biotin is also helpful in maintaining a steady blood sugar level. Biotin is often recommended for strengthening hair and nails. Consequently, it is found in many cosmetic and health products for the hair and skin.
Deficiency is extremely rare, as intestinal bacteria generally produce an excess of the body's daily requirement. For that reason, statutory agencies in many countries (e.g., the Australian Department of Health and Aging) do not prescribe a recommended daily intake.
Sources
Dietary
Biotin is widely distributed in a variety of foods, but most often at low concentrations. Estimates are that the typical U.S. diet provides roughly 40 mcg/day. There are only a couple of foods which contain biotin in large amounts, including royal jelly and brewer's yeast. The best natural sources of biotin in human nutrition are swiss chard, tomatoes, romaine lettuce, and carrots. Other great sources include almonds, eggs, onions, cabbage, cucumber, and cauliflower. And good sources includes goat's milk, cow's milk, raspberries, strawberries, halibut, oats, and walnuts. The most important natural sources in feeding nonruminant animals are oilseed meals, alfalfa, and dried yeasts. It is important to note that the biotin content of food varies and can be influenced by factors such as plant variety, season, and yield (endosperm-to-pericarp ratio).[2]
| Age | Biotin (mcg/day) | |
|---|---|---|
| Infants | 0–6 months | 5 |
| 7–12 months | 6 | |
| Children | 1–3 years | 8 |
| 4–8 years | 12 | |
| Males and Females | 9–13 years | 20 |
| 14-18 years | 25 | |
| 19–70 years | 30 | |
| 70+ years | 30 | |
| Pregnant | <18-50 | 30 |
| Lactating | <18-50 | 35 |
- Adequate intake are determined for nutrients when there is insufficient scientific evidence to establish a Recommended Dietary Allowance (RDA). These values are set as goals for individuals to support adequate nutritional status. NOTE: U.S. Food and supplement labels show 30 mcg of biotin as providing only 10% DV (Daily Value) because DVs are based on older and in some instances outdated RDAs for nutrients. Thus, the DV for biotin is 300 mcg even though there is now consensus that 30 mcg is adequate. There is no current Tolerable Upper Limit (UL) set for biotin as research has indicated that high levels of intake by humans has no detrimental effects.[3]
Bioavailability
Studies on the bioavailability of biotin have been conducted in rats and in chicks. From these studies, it was concluded that biotin bioavailability may be low or variable depending on the type of food being consumed, but in general, approximately half of the biotin in most foods is considered to be biologically available. The biotin present in corn is readily available; however, most grain have about a 20-40% bioavailability of biotin [2].
A possible explanation for the wide variability in biotin bioavailability is that it is due to ability of an organism to break various biotin-protein bonds from food. Whether an organism has an enzyme with the ability to break that bond will determine the bioavailability of biotin from the foodstuff [2].
Factors that Affect Biotin Requirements
The frequency of marginal biotin status is not known, but the incidence of low circulating biotin levels in alcoholics has been found to be much greater than in the general population. Also, relatively low levels of biotin have been reported in the urine or plasma of patients who have had partial gastrectomy or who have other causes of achlorhydria, burn patients, epileptics, elderly individuals and athletes.[2] Pregnancy and lactation may be associated with an increased demand for biotin. In pregnancy, this may be due to a possible acceleration of biotin catabolism, whereas in lactation, the higher demand has yet to be elucidated. Recent studies have shown that marginal biotin deficiency can be present in human gestation, as evidenced by increased urinary excretion of 3-hydroxyisovaleric acid, decreased urinary excretion of biotin and bisnorbiotin, and decreased plasma concentration of biotin. Additionally, smoking may further accelerate biotin catabolism in women.[4]
Uses
Hair Problems
Biotin supplements are often recommended as a natural product to counteract the problem of hair loss in both children and adults. The signs and symptoms of biotin deficiency include hair loss which progresses in severity to include loss of eye lashes and eye brows in severely deficient subjects. Some shampoos are available that contain biotin, but it is doubtful whether they would have any useful effect, as biotin is not absorbed well through the skin.
Cradle cap (seborrheic dermatitis)
Children with a rare inherited metabolic disorder called phenylketonuria (PKU; in which one is unable to break down the amino acid phenylalanine) often develop skin conditions such as eczema and seborrheic dermatitis in areas of the body other than the scalp. The scaly skin changes that occur in people with PKU may be related to poor ability to use biotin. Increasing dietary biotin has been known to improve seborrheic dermatitis in these cases.
Diabetes
People with type 2 diabetes often have low levels of biotin. Biotin may be involved in the synthesis and release of insulin. Preliminary studies in both animals and people suggest that biotin may help improve blood glucose control in those with diabetes, particularly type 2 diabetes.[5] Specifically, biotin doses in excess of nutritional requirements lower postprandial glucose and improve glucose tolerance.[2]
Deficiency
Biotin deficiency is relatively rare and mild, and can be addressed with supplementation. Such deficiency can be caused by the excessive consumption of raw egg whites, which contain high levels of the protein avidin, which binds biotin strongly. Avidin is deactivated by cooking, while the biotin remains intact.
Biotinidase deficiency is not due to inadequate biotin, but rather to a deficiency in the enzymes that process it.
Signs of Biotin Deficiency: In general, appetite and growth are decreased. Dermatologic symptoms include dermatitis, alopecia (hair loss) and achromotrichia (absence or loss of pigment in the hair[6]). Perosis (a shortening and thickening of bones) is seen in the skeleton. Fatty Liver and Kidney Syndrome (FLKS) and hepatic steatosis also can occur.[2]
Toxicity
Animal studies have indicated few, if any, effects due to toxic doses of biotin. This may provide evidence that both animals and humans may tolerate doses of at least an order of magnitude greater than each of their nutritional requirements. There are no reported cases of adverse effects from receiving high doses of the vitamin, particularly when used in the treatment of metabolic disorders causing sebhorrheic dermatitis in infants.[7]
Biochemistry
Biotin D(+) is a cofactor responsible for carbon dioxide transfer in several carboxylase enzymes:
- Acetyl-CoA carboxylase alpha
- Acetyl-CoA carboxylase beta
- Methylcrotonyl-CoA carboxylase
- Propionyl-CoA carboxylase
- Pyruvate carboxylase
The attachment of biotin to various chemical sites, called biotinylation, can be used as an important laboratory technique to study various processes including protein localization, protein interactions, DNA transcription and replication. Biotin itself is known to biotinylate histones , but is not found naturally in chromatin. Holocarboxylase synthetase is involved in the binding of biotin.
Biotin binds very tightly to the tetrameric protein avidin (also streptavidin and neutravidin), with a dissociation constant Kd in the order of 10-15 mol/L which is the strongest known protein-ligand interaction, approaching the covalent bond in strength (Bonjour, 1977; Green 1975; and Roth, 1985). This is often used in different biotechnological applications. Until 2005, very harsh conditions were required to break the biotin-streptavidin bond.[8]
Laboratory uses
In the biology laboratory, biotin is often chemically linked, or tagged, to a molecule or protein for biochemical assays. This process is called biotinylation. Since avidins bind preferentially to biotin, biotin-tagged molecules can be extracted from a sample by mixing them with beads with covalently-attached avidin, and washing away anything unbound to the beads.
For example, biotin can be attached to a molecule of interest (e.g. a protein), and this modified molecule will be mixed with a complex mixture of proteins. Avidin or streptavidin beads are added to the mixture, and the biotinylated molecule will bind to the beads. Any other proteins binding to the biotinylated molecule will also stay with the beads. All other unbound proteins can be washed away, and the scientist can use a variety of methods to determine which proteins have bound to the biotinylated molecule.
Biotinylated antibodies are used to capture avidin or streptavidin in both the ELISPOT and ELISA techniques.
Ruminant Nutrition
Ruminal bacteria normally synthesize biotin. Biotin is not extensively metabolized in the rumen and increased intake of dietary biotin results in elevated concentrations of biotin in serum and milk.[9] Unpublished epidemiologic data suggest a negative relationship between serum concentrations of biotin and the incidence of clinical lameness in dairy cattle. Feeding approximately 20 mg/day of supplemental biotin statistically improved measures of hoof health. Currently, insufficient data are available at this time to quantify the requirement for biotin of dairy cattle.
See also
- Biotinylation
- Avidin
- Streptavidin
- NeutrAvidin
References
- ↑ "Medical Jargon". Retrieved on 2008-09-21.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Combs, Gerald F. Jr. (2008). The Vitamins: Fundamental Aspects in Nutrition and Health. San Diego: Elsevier, Inc. ISBN 9780121834937.
- ↑ McGuire M, Beerman KA. Nutritional sciences: from fundamentals to food. California: Thomson Wadsworth, 2007.
- ↑ Bowman, BA and Russell, RM., ed. (2006), "Biotin", Present Knowledge in Nutrition, Ninth Edition, Vol 1, Washington, DC: Internation Life Sciences Institute, ISBN 9781578811984
- ↑ Campbell, R. Keith (November 2006). "A Critical Review of Chromium Picolinate and Biotin". U.S. Pharmacist 31 (11). http://www.uspharmacist.com/index.asp?show=article&page=8_1895.htm.
- ↑ biology-online.org
- ↑ Combs, Gerald F. Jr. (1998). The Vitamins: Fundamental Aspects in Nutrition and Health. Ithaca: Elsevier Academic Press. ISBN 0121834921.pg. 360
- ↑ Holmberg A, Blomstergren A, Nord O et al. (2005). "The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures". Electrophoresis 26 (3): 501–10. doi:. PMID 15690449.
- ↑ National Research Council (2001). Nutrient Requirements of Dairy Cattle. 7th rev. ed.. Natl. Acad. Sci., Washington, DC.. ISBN 0309069971. http://books.nap.edu/openbook.php?isbn=0309069971.
External links
- Jane Higdon, "Biotin", Micronutrient Information Center, Linus Pauling Institute
- Biotin - Biocytin (Brewer's YeastBiotin Complex)
- Clercq, Pierre J. De (1997). "Biotin: A Timeless Challenge for Total Synthesis". Chemical Review 97: 1755–1792. doi:.
|
|||||||||||||||||||||||||||
|
||||||||||||||