Benign prostatic hyperplasia
| Benign prostatic hyperplasia Classification and external resources |
|
 |
|
|---|---|
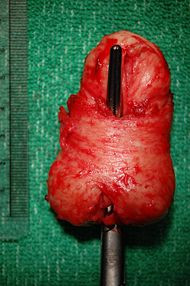
| Prostate with a large median lobe bulging upwards. A metal instrument is placed in the urethra (which passes through the prostate). This specimen was almost 7 centimeters long with a volume of about 60 cubic centimetres on transrectal ultrasound and was removed during a Hryntschak procedure or transvesical prostatectomy (removal of the prostate through the bladder) for benign prostatic hyperplasia. | |
| ICD-10 | N40. |
| ICD-9 | 600 |
| DiseasesDB | 10797 |
| eMedicine | med/1919 |
| MeSH | D011470 |
Benign prostatic hyperplasia (BPH) also known as nodular hyperplasia, benign prostatic hypertrophy (technically a misnomer) or benign enlargement of the prostate (BEP) refers to the increase in size of the prostate in middle-aged and elderly men. To be accurate, the process is one of hyperplasia rather than hypertrophy, but the nomenclature is often interchangeable, even amongst urologists. It is characterized by hyperplasia of prostatic stromal and epithelial cells, resulting in the formation of large, fairly discrete nodules in the periurethral region of the prostate. When sufficiently large, the nodules compress the urethral canal to cause partial, or sometimes virtually complete, obstruction of the urethra which interferes the normal flow of urine. It leads to symptoms of urinary hesitancy, frequent urination, increased risk of urinary tract infections and urinary retention. Although prostate specific antigen levels may be elevated in these patients because of increased organ volume and inflammation due to urinary tract infections, BPH is not considered to be a premalignant lesion.
Adenomatous prostatic growth is believed to begin at approximately age 30 years. An estimated 50% of men have histologic evidence of BPH by age 50 years and 75% by age 80 years. In 40-50% of these patients, BPH becomes clinically significant.[1]
Contents |
Symptoms
Benign prostatic hyperplasia symptoms are classified as obstructive or irritative. Obstructive symptoms include hesitancy, intermittency, incomplete voiding, weak urinary stream, and straining.
Irritative symptoms include frequency of urination, which is called nocturia when occurring at night time, and urgency (compelling need to void that can not be deferred). These obstructive and irritative symptoms are evaluated using the International Prostate Symptom Score (IPSS) questionnaire, designed to assess the severity of BPH.[2]
BPH can be a progressive disease, especially if left untreated. Incomplete voiding results in stasis of bacteria in the bladder residue and an increased risk of urinary tract infections. Urinary bladder stones, are formed from the crystallisation of salts in the residual urine. Urinary retention, termed acute or chronic, is another form of progression. Acute urinary retention is the inability to void, while in chronic urinary retention the residual urinary volume gradually increases, and the bladder distends. Some patients who suffer from chronic urinary retention may eventually progress to renal failure, a condition termed obstructive uropathy.
Etiology
Androgens (testosterone and related hormones) are considered to play a permissive role in BPH by most experts. This means that androgens have to be present for BPH to occur, but do not necessarily directly cause the condition. This is supported by the fact that castrated boys do not develop BPH when they age, unlike intact men. Additionally, administering exogenous testosterone is not associated with a significant increase in the risk of BPH symptoms. Dihydrotestosterone (DHT), a metabolite of testosterone is a critical mediator of prostatic growth. DHT is synthesized in the prostate from circulating testosterone by the action of the enzyme 5α-reductase, type 2. This enzyme is localized principally in the stromal cells; hence, these cells are the main site for the synthesis of DHT.
DHT can act in an autocrine fashion on the stromalie cells or in paracrine fashion by diffusing into nearby epithelial cells. In both of these cell types, DHT binds to nuclear androgen receptors and signals the transcription of growth factors that are mitogenic to the epithelial and stromal cells. DHT is 10 times more potent than testosterone because it dissociates from the androgen receptor more slowly. The importance of DHT in causing nodular hyperplasia is supported by clinical observations in which an inhibitor of 5α-reductase is given to men with this condition. Therapy with 5α-reductase inhibitor markedly reduces the DHT content of the prostate and in turn reduces prostate volume and, in many cases, BPH symptoms.
There is growing evidence that estrogens play a role in the etiology of BPH. This is based on the fact that BPH occurs when men generally have elevated estrogen levels and relatively reduced free testosterone levels, and when prostate tissue becomes more sensitive to estrogens and less responsive to DHT. Cells taken from the prostates of men who have BPH have been shown to grow in response to high estradiol levels with low androgens present. Estrogens may render cells more susceptible to the action of DHT.
On a microscopic level, BPH can be seen in the vast majority of men as they age, particularly over the age of 70 years, around the world. However, rates of clinically significant, symptomatic BPH vary dramatically depending on lifestyle. Men who lead a western lifestyle have a much higher incidence of symptomatic BPH than men who lead a traditional or rural lifestyle. This is confirmed by research in China showing that men in rural areas have very low rates of clinical BPH, while men living in cities adopting a western lifestyle have a skyrocketing incidence of this condition, though it is still below rates seen in the West.
Much work remains to be done to completely clarify the causes of BPH.
Diagnosis

Rectal examination (palpation of the prostate through the rectum) may reveal a markedly enlarged prostate, usually affecting the middle lobe.
Often, blood tests are performed to rule out prostatic malignancy: elevated prostate specific antigen (PSA) levels needs further investigations such as reinterpretation of PSA results, in terms of PSA density and PSA free percentage, rectal examination and transrectal ultrasonography. These combined measures can provide early cancer detection.
Ultrasound examination of the testicles, prostate and kidneys is often performed, again to rule out malignancy and hydronephrosis.
Screening and diagnostic procedures for BPH are similar to those used for Prostate Cancer. Some signs to look for include[3]:
- Weak urinary stream
- Prolonged emptying of the bladder
- Abdominal straining
- Hesitancy
- Irregular need to urinate
- incomplete bladder emptying
- Post-urination dribble
- Irritation during urination
- Frequent urination
- Nocturia– need to urinate during the night
- Urgency
- Incontinence-involuntary leakage of urine.
- Bladder pain
- Dysuria– painful urination
Epidemiology
The prostate gets larger in most men as they get older, and overall, 45% of men over the age of 46 can expect to suffer from the symptoms of BPH if they survive 30 years. Incidence rates increase from 3 cases per 1000 man-years at age 45-49 years, to 38 cases per 1000 man-years by the age of 75-79 years. Whereas prevalence rates are 2.7% for men aged 45-49, increasing to 24% by the age of 80 years.[4]
For some men, the symptoms may be severe enough to require treatment.
Treatment
Lifestyle
Patients should decrease fluid intake before bedtime, moderate the consumption of alcohol and caffeine-containing products, and follow timed voiding schedules.
Medications
Alpha blockers (α1-adrenergic receptor antagonists) provide symptomatic relief of BPH symptoms. Available drugs include doxazosin, terazosin, alfuzosin and tamsulosin. Older drugs, phenoxybenzamine and prazosin are not recommended for treatment of BPH.[5] Alpha-blockers relax smooth muscle in the prostate and the bladder neck, and decrease the degree of blockage of urine flow. Alpha-blockers may cause ejaculation back into the bladder (retrograde ejaculation).
The 5α-reductase inhibitors (finasteride dutasteride) are another treatment option. This medication inhibits 5a-reductase, which in turn inhibits production of DHT, a hormone responsible for enlarging the prostate. When used together with alpha blockers a reduction of BPH progression to acute urinary retention and surgery has been noted in patients with larger prostates.[6]
Though former research indicated the efficacy of Serenoa repens (saw palmetto) fruit extracts in alleviating mild-to-moderate BPH symptoms,[7] a recent double-blind study did not demonstrate any efficacy greater than that of a placebo for moderate-to-severe symptoms.[8] Herbal medicines that have research support in systematic reviews include beta-sitosterol from Hypoxis rooperi (African star grass) and pygeum (extracted from the bark of Prunus africana), while there is less substantial support for the efficacy of Cucurbita pepo (pumpkin) seed and Urtica dioica (stinging nettle) root.[9] At least one double-blind trial has also supported the efficacy of rye flower pollen.[10]
Sildenafil shows some symptomatic relief, suggesting a possible common etiology with erectile dysfunction.[11]
Minimally Invasive Therapies
While medication is often prescribed as the first treatment option, there are many patients who do not achieve success with this line of treatment. For many patients medication is a good option. however patients may not respond to medical management, they may not achieve sustained improvement in symptoms or they may stop taking the medication because of side effects.[12] There are options for treatment in an urologist's office before proceeding to surgery. The two most common types of office-based therapies are Transurethral microwave thermotherapy (TUMT) and TransUrethral Needle Ablation (TUNA). Both of these procedures rely on delivering enough energy to create sufficient heat to cause cell death (necrosis) in the prostate. The goal of the therapies is to cause enough necrosis so that when the dead tissue is reabsorbed by the body the prostate shrinks, relieving the obstruction of the urethra. These procedures are typically performed with local anesthesia, and the patient returns home the same day. Some urologists have studied and published long term data on the outcomes of these procedures, with data out to five years. The most recent American Urological Association (AUA) Guidelines for the Treatment of BPH in 2003 lists minimally invasive therapies including TUMT and TUNA as acceptable alternatives for certain patients with BPH.[13]
Transuretheral microwave therapy (TUMT) was originally approved by the FDA in 1996 with the first generation system by EDAP Technomed. Since 1996 other companies have received FDA approval for TUMT devices, including Urologix, Dornier, Thermatrix, Celsion and Prostalund. Multiple clinical studies have been published on TUMT. The general principle underlying all the devices is that a microwave antenna that resides in a urethral catheter is placed in the intraprostatic area of the urethra. The catheter is connected to a control box outside of the patient's body and is energized to emit microwave radiation into the prostate to heat the tissue and cause necrosis. It is a one-time treatment that takes approximately 30 minutes to 1 hour, depending on the system used. It takes approximately 4 to 6 weeks for the damaged tissue to reabsorb into the patient's body. Some of the devices incorporate circulating coolant through the treatment area with the intent of preserving the urethra while the microwave energy heats the prostatic tissue surrounding the urethra.
Transuretheral needle ablation (TUNA) operates with a different type of energy, radio frequency (RF) energy, but is designed along the same premise as TUMT devices, that the heat the device generates will cause necrosis of the prostatic tissue and shrink the prostate. The TUNA device is inserted into the urethra using a rigid scope much like a cystoscope. The energy is delivered into the prostate using two needles that emerge from the sides of the device, through the urethral wall and into the prostate. The needle-based ablation devices are very effective at heating a localized area to a high enough temperature to cause necrosis. The treatment is typically performed in one session, but may require multiple sticks of the needles depending on the size of the prostate.
Surgery
If medical treatment fails, and the patient elects not to try office based therapies or the physician determines the patient is a better candidate for surgery transurethral resection of prostate (TURP) surgery may need to be performed. TURP is still generally considered the Gold Standard of prostate interventions for patients who require a procedure. This involves removing (part of) the prostate through the urethra. There are also a number of new methods for reducing the size of an enlarged prostate, some of which have not been around long enough to fully establish their safety or side effects. These include various methods to destroy or remove part of the excess tissue while trying to avoid damaging what's left. Transurethral electrovaporization of the prostate (TVP), laser TURP, visual laser ablation (VLAP), ethanol injection, and others are studied as alternatives.
Newer techniques involving lasers in urology have emerged in the last 5-10 years, starting with the VLAP technique involving the Nd:YAG laser with contact on the prostatic tissue. A similar technology called Photoselective Vaporization of the Prostate (PVP) with the GreenLight (KTP) laser have emerged very recently. This procedure involves a high powered 80 Watt KTP laser with a 550 micrometre laser fiber inserted into the prostate. This fiber has an internal reflection with a 70 degree deflecting angle. It is used to vaporize the tissue to the prostatic capsule. KTP lasers target haemoglobin as the chromophore and typically have a penetration depth of 2.0mm (four times deeper than holmium).
Another procedure termed Holmium Laser Ablation of the Prostate (HoLAP) has also been gaining acceptance around the world. Like KTP the delivery device for HoLAP procedures is a 550um disposable side-firing fiber that directs the beam from a high powered 100 Watt laser at a 70degree from the fiber axis. The holmium wavelength is 2,140nm, which falls within the infrared portion of the spectrum and is invisible to the naked eye. Where KTP relies on haemoglobin as a chromophore, water within the target tissue is the chromophore for Holmium lasers. The penetration depth of Holmium lasers is <0.5mm avoiding complications associated with tissue necrosis often found with the deeper penetration and lower peak powers of KTP.
Both wavelengths, KTP and Holmium, ablate approximately one to two grams of tissue per minute.
Post surgery care often involves placement of a Foley Catheter or a temporary Prostatic stent to allow healing and urine to drain from the bladder.
See also
- Prostate
- Prostate cancer
- Prostate specific antigen
- Prostatectomy
- Urinary retention
- Uvula of urinary bladder
References
- ↑ eMedicine - Transurethral Microwave Thermotherapy of the Prostate (TUMT) : Article by Jonathan Rubenstein
- ↑ Barry MJ, Fowler FJ Jr, O'Leary MP, et al (1992). The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148(5): 1549-57. PMID 1279218
- ↑ PreventProstateCancer.info: A Brief Overview of Benign Prostatic Hyperplasia (BPH) [1] (
- ↑ Verhamme KM, Dieleman JP, Bleumink GS, et al (2002). "Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care--the Triumph project". Eur. Urol. 42 (4): 323–8. doi:. PMID 12361895.
- ↑ "AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations". J. Urol. 170 (2 Pt 1): 530–47. 2003. doi:. PMID 12853821.
- ↑ Kaplan SA, McConnell JD, Roehrborn CG, et al (2006). "Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 ml or greater". J. Urol. 175 (1): 217–20; discussion 220–1. doi:. PMID 16406915.
- ↑ Wilt T, Ishani A, Mac Donald R (2002). "Serenoa repens for benign prostatic hyperplasia". Cochrane Database Syst Rev (3): CD001423. PMID 12137626.
- ↑ Bent S, Kane C, Shinohara K, et al (2006). "Saw palmetto for benign prostatic hyperplasia". N. Engl. J. Med. 354 (6): 557–66. doi:. PMID 16467543.
- ↑ Wilt TJ, Ishani A, Rutks I, MacDonald R (2000). "Phytotherapy for benign prostatic hyperplasia". Public Health Nutr 3 (4A): 459–72. PMID 11276294.
- ↑ Buck AC, Cox R, Rees RW, Ebeling L, John A (1990). "Treatment of outflow tract obstruction due to benign prostatic hyperplasia with the pollen extract, cernilton. A double-blind, placebo-controlled study". Br J Urol 66 (4): 398–404. PMID 1699628.
- ↑ McVary KT, Monnig W, Camps JL, Young JM, Tseng LJ, van den Ende G (2007). "Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial". J. Urol. 177 (3): 1071–7. doi:. PMID 17296414.
- ↑ Table 5. Roehrborn CG.Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol. 2008 Winter;10(1):14-25. PMID: 18470272
- ↑ AUA Clinical guidelines for management of BPH
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||