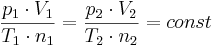Avogadro's law

Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) is a gas law named after Amedeo Avogadro, who in 1811 hypothesized that:
Equal volumes of ideal or perfect gases, at the same temperature and pressure, contain the same number of particles, or molecules.''
Thus, the number of molecules in a specific volume of gas is independent of the size or mass of the gas molecules.
As an example, equal volumes of molecular hydrogen and nitrogen would contain the same number of molecules, as long as they are at the same temperature and pressure and observe ideal or perfect gas behavior. Whilst this is not the real world case, it is statistically very close.
The minor aspect of the law can be stated mathematically as:
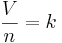 .
.
where:
- V is the volume of the gas.
- n is the number of moles in the gas.
- k is a proportionality constant.
However, this above equation is just a trivial one, which is valid for all homogeneous substances, including homogeneous liquids and solids. This relation is easy to deduce; its validity was assumed before Avogadro's work.
The most important consequence of Avogadro's law is the following: The ideal gas constant has the same value for all gases. This means that the constant
where:
- p is the pressure of the gas
- T is the temperature of the gas
has the same value for all gases, independent of the size or mass of the gas molecules. This statement is nontrivial, and it embodies Avogadro's ingenious insight into the nature of ideal gases. It took decades to prove Avogadro's law based on the kinetic theory of gases.
One mole of an ideal gas occupies 22.4 liters (dm³) at STP, and occupies 24.45 litres at SATP (Standard Ambient Temperature and Pressure = 25 degrees C and 1 atm/101.3kPa). This volume is often referred to as the molar volume of an ideal gas. Real gases may deviate from this value.
The number of molecules in one mole is called Avogadro's number: approximately 6.022×1023 particles per mole.
Avogadro's law, together with the combined gas law, forms the ideal gas law.