Atorvastatin
 |
|
 |
|
|
Atorvastatin
|
|
| Systematic (IUPAC) name | |
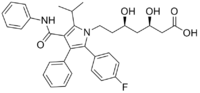
| [R-(R*, R*)]-2-(4-fluorophenyl)-beta, delta-dihydroxy-5- (1-methylethyl)-3-phenyl-4- [(phenylamino)carbonyl]-1H- pyrrole-1-heptanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | C10 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | C33H35FN2O5 |
| Mol. mass | 558.64 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Liver |
| Half life | 14 hours |
| Excretion | Bile |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | oral |
Atorvastatin (INN) (pronounced /əˌtɔrvəˈstætən/), (marketed under the name Lipitor, Lipidra, Aztor, Torvatin, Sortis, Torvast, Torvacard, Totalip, Tulip, Xarator, Atorpic, Liprimar, Atorlip, Avas, Storvas and other names), is a member of the drug class known as statins, used for lowering blood cholesterol. Atorvastatin inhibits HMG-CoA reductase, the rate-determining enzyme located in hepatic tissue that produces mevalonate, a small molecule used in the synthesis of cholesterol and other mevalonate derivatives. This lowers the amount of cholesterol produced which in turn lowers the total amount of LDL cholesterol.
Atorvastatin was first synthesized in 1985 by Bruce Roth while working at Warner-Lambert Company (now Pfizer).
With 2006 sales of US$12.9 billion under the brand name Lipitor, it is the largest selling drug in the world.[1]
Lipitor is not the only statin; there are several other statins on the market.[2][3]
Contents |
Pharmacology
As with other statins, atorvastatin is a competitive inhibitor of HMG-CoA reductase. Unlike most others, however, it is a completely synthetic compound. HMG-CoA reductase catalyzes the reduction of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonate, which is the rate-limiting step in hepatic cholesterol biosynthesis. Inhibition of the enzyme decreases de novo cholesterol synthesis, increasing expression of low-density lipoprotein receptors (LDL receptors) on hepatocytes. This increases LDL uptake by the hepatocytes, decreasing the amount of LDL-cholesterol in the blood. Like other statins, atorvastatin also reduces blood levels of triglycerides and slightly increases levels of HDL-cholesterol.
In clinical trials, adding ezetimibe (Zetia) to Lipitor lowered cholesterol more effectively than Vytorin (ezetimibe + simvastatin).
Clinical use
Indications
Atorvastatin is indicated as an adjunct to diet for the treatment of dyslipidemia, specifically hypercholesterolaemia. It has also been used in the treatment of combined hyperlipidemia.[4]
In 2005, the PROVE IT-TIMI 22 trial compared 40mg/day pravastatin with 80mg/day atorvastatin.[5] Taken directly from the results of the trial: "Standard treatment (statin) with a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (pravastatin 40 mg/day) resulted in a 22% reduction in LDL cholesterol levels at 30 days compared with a 51% reduction with intensive therapy (atorvastatin 80 mg/day). At 2 years, a relative risk reduction of 16% (95% confidence interval, 5%-26%; P = 0.005) in the primary end point rate (death, myocardial infarction, documented unstable angina requiring hospitalization, coronary revascularization, or stroke) was seen in patients receiving intensive statin treatment compared with standard statin therapy. The benefit of intensive treatment was apparent as early as 30 days and was consistent over time. The PROVE IT-TIMI 22 data indicate that patients recently hospitalized for an ACS benefit from early and continued lowering of LDL cholesterol to levels substantially below current guideline recommendations."
Available forms

Atorvastatin calcium tablets are currently marketed by Pfizer under the trade name Lipitor, in tablets (10, 20, 40 or 80 mg) for oral administration. Tablets are white, elliptical, and film coated. Pfizer also packages the drug in combination with other drugs, such as is the case with its Caduet.
Adverse effects
Common adverse drug reactions (≥1% of patients, or more than one out of every one hundred patients) associated with atorvastatin therapy include: myalgia, mild transient gastrointestinal symptoms (diarrhea, constipation, passing gas), elevated hepatic transaminase concentrations, headache, insomnia, joint pain, and/or dizziness.[4]
Myopathy and rhabdomyolysis occur in <0.1% of patients (less than 1 out of every 1,000 patients). Risk is increased in patients with renal impairment, serious concurrent illness; and/or concomitant use of drugs which inhibit CYP3A4.[4]
Market
Patent challenge
The size of the market for atorvastatin has prompted the generic drug manufacturing company Ranbaxy to challenge the validity of some of Pfizer's patents in patent courts across the world. As of March 2007, courts had mostly upheld the validity of Pfizer's original patent for atorvastatin, which is due to expire in European territories in 2011 (but 2010 in Canada). However a later patent for the specific enantiomer of the atorvastatin formula that is medically useful, which would have given Pfizer longer protection, has fared less well. Although upheld in the United States[6], Spain, and Ecuador, the enantiomer patent has been declared invalid by courts in Malaysia, Austria, Australia, Canada, the Netherlands and the United Kingdom[7].
Pfizer fight against simvastatin alternative
After doctors and patients began switching by the millions to a cheaper alternative within the same class of drugs called simvastatin, Pfizer launched a campaign including advertisements, lobbying efforts, and a paid speaking tour by Dr. Louis W. Sullivan, a former secretary of the federal Department of Health and Human Services, to discourage the trend.[2] The main clinical advantage of Lipitor over Simvastatin is that it is not metabolised by certain liver enzymes, and thus its blood concentration is not increased when combined with grapefruit juice which inhibits these enzymes. Simvastatin patients should avoid drinking large amounts of grapefruit juice for this reason. An independent analysis showed that, at commonly prescribed doses, atorvastatin and simvastatin have no statistically significant differences in reducing cardiovascular morbidity and mortality.[8]
Advertisements withdrawn
On February 25, 2008, Pfizer announced that it will voluntarily withdraw all advertisements for Lipitor featuring Dr. Robert Jarvik, and committing to ensuring greater clarity in the roles and responsibilities of its spokespeople in its consumer advertising and promotion. [9] Dr. Jarvik is not a licensed physician and his use in advertisements was considered misleading. In addition, although he was shown rowing a shell (and therefore implying that his heart was healthy) he in fact does not row and the advertisement employed a double. Pfizer withdrew the advertisement as a result of bad press.
References
- ↑ Loftus, Peter (2007-08-16). "Pfizer's Lipitor Patent Reissue Rejected", The Wall Street Journal Online. Retrieved on 2007-08-27.
- ↑ 2.0 2.1 Saul, Stephanie; Alex Berenson (2007-11-03). "Maker of Lipitor Digs In to Fight Generic Rival". The New York Times.
- ↑ Hawkes, Nigel (2007-10-11). "Statins are the right prescription". The Times (UK). Retrieved on 2007-11-03.
- ↑ 4.0 4.1 4.2 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Rouleau J (2005). "Improved outcome after acute coronary syndromes with an intensive versus standard lipid-lowering regimen: results from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial". Am. J. Med. 118 Suppl 12A: 28–35. doi:. PMID 16356805.
- ↑ BBC News, Patent ruling hits Ranbaxy shares, 19 December 2005
- ↑ Duncan Bucknell, The global Lipitor patent scorecard, retrieved 2007-03-03
- ↑ Zhou Z, Rahme E, Pilote L (2006). "Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention". Am. Heart J. 151 (2): 273–81. doi:. PMID 16442888.
- ↑ "Drugs.com, Pfizer Voluntarily Withdraws Lipitor Advertising Featuring Dr. Robert Jarvik". Retrieved on 2008-03-08.
External links
- Lipitor.com – manufacturer's site
- MedlinePlus Drug information: Atorvastatin (Systemic) – information from USP DI Advice for the Patient
Further reading
- Maggon K (2005). "Best-selling human medicines 2002–2004". Drug Discov. Today 10 (11): 739–42. doi:. PMID 15922927.
- Lipitor: Prescribing Information. (2004) Pfizer Ireland Pharmaceuticals.
- Bruce D. Roth, The Discovery and Development of Atorvastatin, a Potent Novel Hypolipidemic Agent, Progress in Medical Chemistry, 2002, pp. 1–22, vol. 40.
- The $10 Billion Pill - 2003 FORTUNE article on Bruce Roth
- The Birth of a Blockbuster: Lipitor's Route out of the Lab - Wall Street Journal
- Ann Arbor chemist wins national award for drug discovery - ScienceBlog
- Meet the Guy Who Invented Lipitor - Wired article
- Chemical Society To Honor 'Heroes Of Chemistry' During National Meeting
- Bruce D. Roth, Pfizer Inc, USA
|
||||||||||||||||||||||||||