Astatine
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | astatine, At, 85 | |||||||||||||||||||||
| Element category | halogens | |||||||||||||||||||||
| Group, Period, Block | 17, 6, p | |||||||||||||||||||||
| Appearance | black solid (presumed) | |||||||||||||||||||||
| Standard atomic weight | (210) g·mol−1 | |||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p5 | |||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 7 | |||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||
| Melting point | 575 K (302 °C, 576 °F) |
|||||||||||||||||||||
| Boiling point | ? 610 K (? 337 °C, ? 639 °F) |
|||||||||||||||||||||
| Heat of vaporization | ca. 40 kJ·mol−1 | |||||||||||||||||||||
|
||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||
| Crystal structure | no data | |||||||||||||||||||||
| Oxidation states | ±1, 3, 5, 7 | |||||||||||||||||||||
| Electronegativity | 2.2 (Pauling scale) | |||||||||||||||||||||
| Ionization energies | 1st: 890±40 kJ/mol | |||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||
| Magnetic ordering | no data | |||||||||||||||||||||
| Thermal conductivity | (300 K) 1.7 W·m−1·K−1 | |||||||||||||||||||||
| CAS registry number | 7440-68-8 | |||||||||||||||||||||
| Most-stable isotopes | ||||||||||||||||||||||
|
||||||||||||||||||||||
| References | ||||||||||||||||||||||
Astatine (pronounced /ˈæstətiːn/; ass'-ta-tyne) is a radioactive chemical element with the symbol At and atomic number 85. It is the heaviest of the discovered halogens. Although astatine is produced by radioactive decay in nature, due to its short half life it is found only in minute amounts. Astatine was first produced by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè in 1940. Three years passed before traces of astatine were also found in natural minerals. Until recently most of the physical and chemical characteristics of astatine were inferred by comparison to other elements. The alpha-emitting properties of some astatine isotopes are used for science applications, and also medical applications for astatine 211 are tested.
Contents |
Characteristics
This highly radioactive element has been confirmed by mass spectrometers to behave chemically much like other halogens, especially iodine (it would probably accumulate in the thyroid gland like iodine[1]), though astatine is thought to be more metallic than iodine. Researchers at the Brookhaven National Laboratory have performed experiments that have identified and measured elementary reactions that involve astatine; however, chemical research into astatine is limited by its extreme rarity, which is a consequence of its extremely short half-life. Its most stable isotope has a half-life of around 8.3 hours. The final products of the decay of astatine are isotopes of lead. Following the color trend of the halogens, the elements get darker in color with increasing molecular weight and atomic number. Thus, following the trend, astatine would be expected to be a nearly black solid, which, when heated, sublimes into a dark, purplish vapor (darker than iodine). Astatine is expected to form ionic bonds with metals such as sodium, like the other halogens, but it can be displaced from the salts by lighter, more reactive halogens. Astatine can also react with hydrogen to form hydrogen astatide, which when dissolved in water, forms hydroastatic acid. Astatine is the least reactive of the halogens, being less reactive than iodine.[2]
History
The existence of "eka-iodine" had been predicted by Mendeleev. Astatine (after Greek αστατος astatos, meaning "unstable") was first synthesized in 1940 by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè at the University of California, Berkeley by bombarding bismuth with alpha particles.[3]
As the periodic table of elements was long known, several scientists tried to find the element following iodine in the halogen group. The unknown substance was called Eka-iodine before its discovery because the name of the element was to be suggested by the discoverer. The claimed discovery in 1931 at the Alabama Polytechnic Institute (now Auburn University) led to the name for the element alabamine (Ab).[4][5][6].
The name Dakin was proposed for this element in 1937 by chemist Rajendralal De working in Dhaka.[7]
The name Helvetium was chosen by the Swiss chemist Walter Minder, when he announced the discovery of element 85 in 1940, but changed his suggested name to Anglohelvetium in 1942.[8]
It took three years before astatine was found as product of the natural decay processes. The short lived element was found by the two scientists Berta Karlik and Traude Bernert.[9][10]
Occurrence
Astatine occurs naturally in three natural radioactive decay series, but because of its short half-life is found only in minute amounts. Astatine-218 (218At) is found in the uranium series, 216At is in the thorium series, and 215At as well as 219At are in the actinium series[11]. The most long-lived of these naturally-occurring astatine isotopes is 210At with a half-life of 8.3 hours.
Astatine is the rarest naturally-occurring element, with the total amount in Earth's crust estimated to be less than 1 oz (28 g) at any given time. This amounts to less than one teaspoon of the element. Guinness World Records has dubbed the element the rarest on Earth, stating: "Only around 0.9 oz (25 g) of the element astatine (At) occurring naturally". Isaac Asimov, in a 1957 essay on large numbers, scientific notation, and the size of the atom, wrote that in "all of North and South America to a depth of ten miles", the number of astatine atoms at any time is "only a trillion".[12]
Production
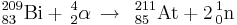 for alpha particles of 26 MeV[13]
for alpha particles of 26 MeV[13]
 for alpha particles of 40 MeV[13]
for alpha particles of 40 MeV[13]
 for alpha particles of 60 MeV.[14]
for alpha particles of 60 MeV.[14]
Astatine is produced by bombarding bismuth with energetic alpha particles to obtain relatively long-lived 209At - 211At, which can then be distilled from the target by heating in the presence of air.
Compounds
Multiple compounds of astatine have been synthesized in microscopic amounts and studied as intensively as possible before their inevitable radioactive disintegration. The reactions are normally tested with dilluted solutions of astatine and an iodine mixtures. The iodine is the carrier and ensures that enough material is there that laboratory techniques, like filtration and the formation of precipitates work.[13][15]
While these compounds are primarily of theoretical interest, they are being studied for potential use in nuclear medicine.[16] Astatine is expected to form ionic bonds with metals such as sodium, like the other halogens, but it can be displaced from the salts by lighter, more reactive halogens. Astatine can also react with hydrogen to form hydrogen astatide (HAt), which when dissolved in water, forms hydroastatic acid.
Some examples of astatic compounds are:
NaAt or sodium astatide
MgAt2 or magnesium astatide
CAt4 or carbon tetrastatide (tetraastatide)
Isotopes
Astatine has 33 known isotopes, all of which are radioactive; the range of their mass numbers is from 191 to 223. There exist also 23 metastable excited states. The longest-lived isotope is 210At, which has a half-life of 8.1 hours; the shortest-lived known isotope is 213At, which has a half-life of 125 nanoseconds.[17]
Applications
The least stable isotopes of astatine have no practical applications other than scientific study due to their extremely short life, but heavier isotopes have medical uses. Astatine 211 is an alpha emitter with a physical halflife of 7.2 h. These features have led to its use in radiation therapy.[18] An investigation of the efficacy of astatine-211--tellurium colloid for the treatment of experimental malignant ascites in mice reveals that this alpha-emitting radiocolloid can be curative without causing undue toxicity to normal tissue. By comparison, beta-emitting phosphorus-32 as colloidal chromic phosphate had no antineoplastic activity. The most compelling explanation for this striking difference is the dense ionization and short range of action associated with alpha-emission. These results have important implications for the development and use of alpha-emitters as radiocolloid therapy for the treatment of human tumors.[19]
Precautions
Since astatine is radioactive, it should be handled with care. Because of its extreme rarity, it is not likely that the general public will be exposed.
Astatine is a halogen, and standard precautions apply. It is less reactive than iodine, but they share similar characteristics.
References
- ↑ "Astatine". Los Alamos Laboratories. Retrieved on 2008-07-10.
- ↑ Anders, E. (1959). "Technetium and Astatine Chemistry". Annual Review of Nuclear Science 9: 203–220. doi:.
- ↑ D. R. Corson, K. R. MacKenzie, and E. Segrè (1940). "Artificially Radioactive Element 85". Phys. Rev. 58: 672–678. doi:.
- ↑ Fred Allison, Edgar J. Murphy, Edna R. Bishop, and Anna L. Sommer (1931). "Evidence of the Detection of Element 85 in Certain Substances". Phys. Rev. 37: 1178–1180. doi:.
- ↑ "Alabamine & Virginium". time. Retrieved on 2008-07-10.
- ↑ Trimble, R. F. (1975). "What happened to alabamine, virginium, and illinium?". J. Chem. Educ. 52: 585.
- ↑ 85 Astatine
- ↑ Alice Leigh-Smith, Walter Minder (1942). "Experimental Evidence of the Existence of Element 85 in the Thorium Family". nature 150: 767–768. doi:.
- ↑ Karlik, Berta; Bernert Traude (1943). "Eine neue natürliche α-Strahlung". Naturwissenschaften 31 (25–26): 298–299. doi:.
- ↑ Karlik, Berta; Bernert Traude (1943). "Das Element 85 in den natürlichen Zerfallsreihen". Zeitschrift für Physik 123 (1–2): 51–72. doi:.
- ↑ "astatine (At)". Encyclopedia Britannica online. Retrieved on 2008-06-22.
- ↑ http://ia331335.us.archive.org/1/items/onlyatrillion017765mbp/onlyatrillion017765mbp_djvu.txt
- ↑ 13.0 13.1 13.2 Nefedov, V. D. (1968). "Astatine". Russ. Chem. Rev. 37: 87–98. doi:.
- ↑ Barton, G. W.; Ghiorso, A.; Perlman, I. (1951). "Radioactivity of Astatine Isotopes". Physical Reviews 82 (1): 13–19. doi:.
- ↑ Aten Jun., A. H. W.; Doorgeest, T.; Hollstein U.; Moeken H. P. (1952). "Section 5: radiochemical methods. Analytical chemistry of astatine". Analyst 77: 774–777. doi:.
- ↑ Boyd, Jade (1007-08-27). "Nuclear Nanocapsules, The New Cancer Weapon". Medical News Today. Retrieved on 2008-11-05.
- ↑ Audi, Georges (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. doi:.
- ↑ Wilbur, D. S. (2001). "Overcoming the Obstacles to Clinical Evaluation of 211At-Labeled Radiopharmaceuticals.". Journal Nuclear Medicine 42: 1516–1518. http://jnm.snmjournals.org/cgi/reprint/42/10/1516.
- ↑ Bloomer, W. D.; McLaughlin, W. H.; Neirinckx, R. D.; Adelstein, S. J.; Gordon, P. R.; Ruth, T. J.; Wolf, AP (1981). "Astatine-211--tellurium radiocolloid cures experimental malignant ascites". Science 212 (4492): 340–341. doi:. http://www.sciencemag.org/cgi/content/abstract/212/4492/340.
External links
- WebElements.com - Astatine
- Los Alamos National Laboratory - Astatine
- Doc Brown's Chemistry Clinic - Group 7 The Halogens
|
|||||
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||