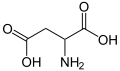
Aspartic acid
| Aspartic acid | |
|---|---|
 |
 |
| IUPAC name | (2S)-2-aminobutanedioic acid |
| Identifiers | |
| CAS number | 56-84-8 |
| PubChem | |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C4H7NO4 |
| Molar mass | 133.1 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Aspartic acid (abbreviated as Asp or D; Asx or B represent either aspartic acid or asparagine)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CO2H. The carboxylate anion of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins. Its codons are GAU and GAC.
Aspartic acid is, together with glutamic acid, classified as an acidic amino acid with a pKa of 4.0. Aspartic acid is pervasive in biosynthesis. As with all amino acids, the location of acid protons depends on the pH of the solution and the crystallization conditions.
Contents |
Role in biosynthesis of amino acids
Aspartic acid is non-essential in mammals, being produced from oxaloacetate by transamination. In plants and microorganisms, aspartic acid is the precursor to several amino acids, including four that are essential: methionine, threonine, isoleucine, and lysine. The conversion of aspartic acid to these other amino acids begins with reduction of aspartic acid to its "semialdehyde," HO2CCH(NH2)CH2CHO.[2] Asparagine is derived from aspartic acid via transamidation:
- HO2CCH(NH2)CH2CO2H + GC(O)NH2 HO2CCH(NH2)CH2CONH2 + GC(O)OH
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively)
Other biochemical roles
Aspartate is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malate-aspartate shuttle, which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartic acid donates one nitrogen atom in the biosynthesis of inositol, the precursor to the purine bases.
Neurotransmitter
Aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter glutamate does.[3] It serves as an excitatory neurotransmitter in the brain and is an excitotoxin.
As a neurotransmitter, aspartic acid may provide resistance to fatigue, and, thus, leads to endurance, although the evidence to support this idea is not strong.
Sources
Dietary Sources
Aspartic acid is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans. Aspartic acid is found in:
- Animal sources: luncheon meats, sausage meat, wild game
- Vegetable sources: sprouting seeds, oat flakes, avocado, asparagus.
- Dietary supplements[4]
Chemical Synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2).[5]
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved on 2007-05-17.
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Philip E. Chen, Matthew T. Geballe, Phillip J. Stansfeld, Alexander R. Johnston, Hongjie Yuan, Amanda L. Jacob, James P. Snyder, Stephen F. Traynelis, and David J. A. Wyllie. 2005. Structural Features of the Glutamate Binding Site in Recombinant NR1/NR2A N-Methyl-D-aspartate Receptors Determined by Site-Directed Mutagenesis and Molecular Modeling. Molecular Pharmacology. Volume 67, Pages 1470-1484.
- ↑ "Emergen-C, Supplementary Facts: Acai Berry (direct link to page not available)" (in English). Retrieved on 26 November 2008.
- ↑ Dunn, M. S.; Smart, B. W. “DL-Aspartic Acid”Organic Syntheses, Collected Volume 4, p.55 (1963). http://www.orgsyn.org/orgsyn/pdfs/CV4P0055.pdf
See also
- Aspartate transaminase
- Sodium poly(aspartate), a synthetic polyamide
|
||||||||||||||