Arsenic trioxide
| Arsenic trioxide | |
|---|---|
 |
|
 |
|
| Other names | Arsenic(III) oxide, Arsenic sesquioxide, Arsenicum album, Arseneous oxide, Arseneous anhydride, White arsenic[1] |
| Identifiers | |
| CAS number | 1327-53-3 |
| PubChem | |
| EINECS number | |
| DrugBank | |
| Properties | |
| Molecular formula | As2O3 |
| Molar mass | 197.841 g/mol |
| Appearance | White solid |
| Density | 3.86 g/cm³, solid (cubic arsenolite form) |
| Melting point |
274°C |
| Boiling point |
460°C |
| Solubility in water | 2 g/100 ml (25°C) see text |
| Acidity (pKa) | 9.2 |
| Structure | |
| Crystal structure | cubic (α)<180°C monoclinic (β) >180°C |
| Molecular shape | See Text |
| Dipole moment | Zero |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−657.4 kJ/mol |
| Standard molar entropy S |
? J.K–1.mol–1 |
| Pharmacology | |
| Protein binding | 75% bound |
| Hazards | |
| EU classification | Very toxic (T+) Carc. Cat. 1 Dangerous for the environment (N) |
| NFPA 704 |
 0
3
2
|
| R-phrases | R45, R28, R34, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| Related compounds | |
| Other anions | Arsenic trisulfide |
| Other cations | Phosphorus trioxide Antimony trioxide |
| Related compounds | Arsenic pentoxide Arsenous acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Arsenic trioxide is the most important commercial compound of arsenic, and the main starting material for arsenic chemistry. It is the highly toxic byproduct of certain kinds of ore processing, for example gold mining.[2] It is found in nature as the minerals arsenolite, cubic and claudetite, monoclinic.
Contents[hide] |
Preparation
- Burning arsenic in air.
- Hydrolysis of arsenic trichloride.
- Roasting of arsenide minerals. (Main industrial route)
Chemical properties
Arsenic trioxide is an amphoteric oxide which shows a marked preponderance for its acidic properties. It dissolves readily in alkaline solutions to give arsenites. It is much less soluble in acids, but will dissolve in hydrochloric acid to give arsenic trichloride or related species. It reacts with oxidizing agents such as ozone, hydrogen peroxide and nitric acid to give arsenic pentoxide, As2O5: the reaction with hydrogen peroxide can be explosive. It is also readily reduced to arsenic, and arsine (AsH3) may also be formed.
Structure
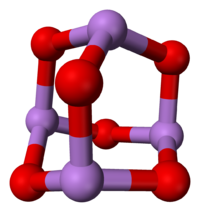
In the liquid and in the gas phase below 800 °C it is As4O6, (isostructural with P4O6).[3] Above 800°C partial dissociation occurs to give molecular As2O3 with the N2O3 structure.[3] In the solid state there are three forms, a cubic form which contains molecular As4O6 and two related monoclinic forms that contain layers of pyramidal AsO3 units sharing O atoms.[3]
Uses
- Starting point for the manufacture of arsenic-based pesticides .(sodium arsenite, sodium arsenate, sodium cacodylate).
- Starting point for the manufacture of certain arsenic-based pharamaceuticals (Neosalvarsan) and veterinary products.
- Decolorizing agent for glasses and enamels.
- Preservative for wood.
- Hydrogen recombination poison for metallurgical studies.
- Termite Poison
- Starting point for the preparation of elemental arsenic, arsenic alloys and arsenide semiconductors.
- Use as a cytostatic in the treatment of refractory promyelocytic (M3) subtype of acute myeloid leukemia. The drug is available as Trisenox ampules; each containing 10mg to be diluted for i.v. infusion.[4]
- Arsenic trioxide is also used to treat leukemia in patients who have not responded to other medications[5]
- In Austria there lived the so called "arsenic eaters", who ingested doses far beyond the lethal dose of arsenic trioxide without any apparent harm. They thought that they would become more powerful for their strenuous work in the Alps. [6][7][8]
- Arsenic trioxide was mixed with copper(II) acetate to form the extremely toxic but exceedingly vibrant pigment known as paris green for its use as a rodenticide in the paris subways.
- Murder [9][10]
- Suicide [11][10]
Medical applications
Arsenic trioxide under the trade name Trisenox (manufacturer: Cephalon) is a chemotheraputic agent of idiopathic function used to treat leukemia that is unresponsive to first line agents. It is suspected that arsenic trisulfide induces cancer cells to undergo apoptosis. Due to the toxic nature of arsenic, this drug carries significant risks.
The combination therapy of arsenic trioxide and all-trans retinoic acid (ATRA) could clearly improve survival rates.
The enzyme thioredoxin reductase has recently been identified as a target for arsenic trioxide.[12]
Toxicology
- See also: arsenicosis.
Arsenic trioxide is readily absorbed by the digestive system: toxic effects are also well known after inhalation of the dust or fumes and after skin contact. Elimination is rapid at first (half-life of 1–2 days), by methylation to cacodylic acid and excretion in the urine, but a certain amount (30–40% in the case of repeated exposure) is incorporated into the bones, muscles, skin, hair and nails (all tissues rich in keratin) and eliminated over a period of weeks or months.
The first symptoms of acute arsenic poisoning by ingestion are digestive problems: vomiting, abdominal pains, diarrhea often accompanied by bleeding. Sub-lethal doses can lead to convulsions, cardiovascular problems, inflammation of the liver and kidneys and abnormalities in the coagulation of the blood. These are followed by the appearance of characteristic white lines (Mees stripes) on the nails and by hair loss. Lower doses lead to liver and kidney problems and to changes in the pigmentation of the skin.
Cases of acute arsenic poisoning are known after inhalation and after skin contact with arsenic trioxide. The first signs are severe irritation, either of the respiratory tract or of the exposed skin, followed by longer term neurological problems. Even dilute solutions of arsenic trioxide are dangerous on contact with the eyes.
Chronic arsenic poisoning is known as arsenicosis: it is found after professional exposure (for example, in metal smelters), in populations whose drinking water contains high levels of arsenic (0.3–0.4 ppm) and in patients treated for long periods with arsenic-based pharmaceuticals.
Arsenic trioxide has been shown to be a human carcinogen. Studies on workers exposed in copper foundries in the U.S., Japan and Sweden indicate a risk of lung cancer 6–10 times higher for the most exposed workers compared with the general population. Long-term ingestion of arsenic trioxide either in drinking water or as a medical treatment can lead to skin cancer. Reproductive problems (high incidence of miscarriage, low birth weight, congenital deformations) have also been indicated in one study of women exposed to arsenic trioxide dust as employees or neighbours of a copper foundry.
Natural occurrence
Two minerals are known to possess the As2O3 chemical formula: arsenolite(regular) and claudetite (monoclinic). Both are relatively rare secondary minerals found in oxidation zones of As-rich ore deposits (these are often Co-, Ni-, Ag- and U-bearing, too).
Bibliography
- Institut national de recherche et de sécurité (INRS), Fiche toxicologique nº 89 : Trioxyde de diarsenic, 1989.
- AFHS Database on use as a cytostatic
References
- ↑ Shakhashiri BZ, "Chemical of the Week: Arsenic", University of Wisconsin-Madison Chemistry Dept.
- ↑ "Giant Mine - Northwest Territories Region - Indian and Northern Affairs Canada". Retrieved on 2007-08-28.
- ↑ 3.0 3.1 3.2 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- ↑ .Steven L. Soignet et al. (2001). "United States Multicenter Study of Arsenic Trioxide in Relapsed Acute Promyelocytic Leukemia". Journal of Clinical Oncology 19 (18): 3852–3860.
- ↑ .Antman, K. H. (2001). "Introduction: The history of arsenic trioxide in cancer therapy". Oncologist 6(Suppl. 2) (1–2): 2006.
- ↑ Arsenic Eaters — New York Times July 26, 1885
- ↑ cf. Richard M. Allesch. Arsenik. Seine Geschichte in Österreich. 54. Band. Klagenfurt: Kleinmayr 1959.
- ↑ G. Przygoda, J. Feldmann, W. R. Cullen (2001). "The arsenic eaters of Styria: a different picture of people who were chronically exposed to arsenic". Applied Organometallic Chemistry 15 (6): 457–462. doi:. http://doi.wiley.com/10.1002/aoc.126.
- ↑ "Stanton v Benzler 9716830". U.S. 9th Circuit Court of Appeals (1998-06-17). Retrieved on 2008-06-09. "(...) convicted by a jury of first degree murder for poisoning her ex-husband. Her ex-husband's body was found with traces of arsenic trioxide in it."
- ↑ 10.0 10.1 Emsley, John (2006). "Arsenic". The Elements of Murder: A History of Poison. Oxford University Press. pp. 93 –197. ISBN 9780192806000.
- ↑ Madame Bovary by Flaubert
- ↑ Lu J, Chew EH, Holmgren A (2007). "Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide". Proc. Natl. Acad. Sci. U.S.A. 104 (30): 12288–93. doi:. PMID 17640917.
External links
- - Solubility of As2O3 in water as function of temperature
- Case Studies in Environmental Medicine: Arsenic Toxicity
- IARC Monograph – Arsenic and Arsenic Compounds
- International Chemical Safety Card 0378
- NIOSH Pocket Guide to Chemical Hazards
- NTP Report on Carcinogens – Inorganic Arsenic Compounds
- Use of Arsenic Trioxide in Multiple Myeloma Treatment
- The use of Arsenic trioxide in medicine.
- Institut national de recherche et de sécurité (1989). "Trioxyde d'arsenic."PDF Fiche toxicologique n° 89. Paris:INRS. (French)
- Institute of Chemistry Austria, speciallised on arsenic and various arsenic compounds
|
||||||||||||||||||||||||||||||||||||||||||||||