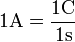Ampere

The ampere, in practice often shortened to amp, (symbol: A) is a unit of electric current, or amount of electric charge per unit time, in coulombs per second. The ampere is an SI base unit, and is named after André-Marie Ampère, one of the main discoverers of electromagnetism.
Contents |
Definition
One ampere is defined to be the constant current which will produce an attractive force of 2×10–7 newton per metre of length between two straight, parallel conductors of infinite length and negligible circular cross section placed one metre apart in a vacuum.[1][2] The definition is based on Ampère's force law.[3] The ampere is a base unit, along with the metre, kelvin, second, mole, candela and the kilogram: it is defined without reference to the quantity of electric charge.
The SI unit of charge, the coulomb, "is the quantity of electricity carried in 1 second by a current of 1 ampere.".[4] Conversely, a current of one ampere is one coulomb of charge going past a given point per second:
that is, in general, charge Q is determined by steady current I flowing for a time t as:
History
The ampere was originally defined as one tenth of the CGS system electromagnetic unit of current (now known as the abampere), the amount of current which generates a force of two dynes per centimetre of length between two wires one centimetre apart.[5] The size of the unit was chosen so that the units derived from it in the MKSA system would be conveniently sized.
The "international ampere" was an early realization of the ampere, defined as the current that would deposit 0.001118000 grams of silver per second from a silver nitrate solution.[6] Later, more accurate measurements revealed that this current is 0.99985 A.
Realization
The ampere is most accurately realized using a watt balance, but is in practice maintained via Ohm's Law from the units of EMF and resistance, the volt and the ohm, since the latter two can be tied to physical phenomena that are relatively easy to reproduce, the Josephson junction and the quantum Hall effect, respectively. The official realization of a standard ampere is discussed in NIST Special publication 330 Barry N Taylor (editor) Appendix 2, p. 56.
Proposed future definition
Since a coulomb is approximately equal to 6.24150948×1018 elementary charges, one ampere is approximately equivalent to 6.24150948×1018 elementary charges, such as electrons, moving past a boundary in one second.
As with other SI base units, there have been proposals to redefine the kilogram in such a way as to define some presently measured physical constants to fixed values. One proposed definition of the kilogram is:
The kilogram is the mass which would be accelerated at precisely 2×10-7 m/s2 if subjected to the per metre force between two straight parallel conductors of infinite length, of negligible circular cross section, placed 1 metre apart in vacuum, through which flow a constant current of exactly 6,241,509,479,607,717,888 elementary charges per second.
This redefinition of the kilogram has the effect of fixing the elementary charge to be e = 1.60217653×10-19 C and would result in a functionally equivalent definition for the coulomb as being the sum of exactly 6 241 509 479 607 717 888 elementary charges and the ampere as being the electrical current of exactly 6 241 509 479 607 717 888 elementary charges per second. This is consistent with the current 2002 CODATA value for the elementary charge which is 1.60217653×10-19 ± 0.00000014×10-19 C.
CIPM recommendation
International Committee for Weights and Measures (CIPM) Recommendation 1 (CI-2005): Preparative steps towards new definitions of the kilogram, the ampere, the kelvin and the mole in terms of fundamental constants
The International Committee for Weights and Measures (CIPM),
- approve in principle the preparation of new definitions and mises en pratique of the kilogram, the ampere and the kelvin so that if the results of experimental measurements over the next few years are indeed acceptable, all having been agreed with the various Consultative Committees and other relevant bodies, the CIPM can prepare proposals to be put to Member States of the Metre Convention in time for possible adoption by the 24th CGPM in 2011;
- give consideration to the possibility of redefining, at the same time, the mole in terms of a fixed value of the Avogadro constant;
- prepare a Draft Resolution that may be put to the 23rd CGPM in 2007 to alert Member States to these activities.
See also
- Ammeter
- Ampère's force law
- Coulomb
- Electric shock
- Hydraulic analogy
- International System of Units
- Magnetic constant
- Ohm's Law
- Watt
References
- ↑ BIPM official definition
- ↑ Paul M. S. Monk, Physical Chemistry: Understanding our Chemical World, John Wiley and Sons, 2004 online.
- ↑ Raymond A Serway & Jewett JW (2006). Serway's principles of physics: a calculus based text (Fourth Edition ed.). Belmont, CA: Thompson Brooks/Cole. p. 746. ISBN 053449143X. http://books.google.com/books?id=1DZz341Pp50C&pg=RA1-PA746&dq=wire+%22magnetic+force%22&lr=&as_brr=0&sig=4vMV_CH6Nm8ZkgjtDJFlupekYoA#PRA1-PA746,M1.
- ↑ Bureau International des Poids et Mesures. (2006).The International System of Units (SI), 8th ed. p. 144.
- ↑ A short history of the SI units in electricity
- ↑ History of the ampere