Ammonium sulfate
| Ammonium sulfate | |
|---|---|
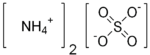 |
|
| IUPAC name | Ammonium sulfate |
| Other names | ammonium sulfate (2:1); diammonium sulfate; sulfuric acid diammonium salt; mascagnite; Actamaster; Dolamin |
| Identifiers | |
| CAS number | 7783-20-2 |
| SMILES |
|
| Properties | |
| Molecular formula | (NH4)2SO4 |
| Molar mass | 132.14052 g/mol |
| Appearance | Fine white hygroscopic granules or crystals. |
| Density | 1.77 g/cm³ @ 50 °C (122 °F) |
| Melting point |
235-280 °C, 508-553 K, 455-536 °F (decomposes) |
| Solubility in water | 70.6 g/100 mL (0 °C) and 103.8 g/100 mL (100 °C)[1] |
| Critical relative humidity | 79.2% at 30 °C |
| Related compounds | |
| Related compounds | Ammonium iron sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Ammonium sulfate, (NH4)2SO4, is an inorganic chemical compound commonly used as a fertilizer. It contains 21% nitrogen as ammonium ions and 24% sulfur as sulfate ions. Ammonium sulfate occurs naturally as the rare mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps.[2]
Contents |
Properties
Ammonium sulfate is not soluble in alcohol or liquid ammonia. The compound is slightly hygroscopic and absorbs water from the air at relative humidity > 81% (at ca. 20°C).
Synthesis
Ammonium sulfate is prepared commercially by reacting ammonia with sulfuric acid (H2SO4). Ammonium sulfate is prepared commercially from the ammoniacal liquor of gas-works and is purified by recrystallisation. It forms large rhombic prisms, has a somewhat saline taste and is easily soluble in water.
Uses
It is used largely as an artificial fertilizer for alkaline soils. In the soil the sulfate ion is released and forms bisulfate, lowering the pH balance of the soil (as do other sulfate compounds such as aluminium sulfate), while contributing essential nitrogen for plant growth.
It is also used as an agricultural spray adjuvant for water soluble insecticides, herbicides, and fungicides. There it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D (amine), glyphosate, and glufosinate herbicides.
It is also used in the preparation of other ammonium salts.
In biochemistry, ammonium sulfate precipitation is a common method for purifying proteins by precipitation. As such, ammonium sulfate is also listed as an ingredient for many United States vaccines per the Center for Disease Control.[3]
Ammonium sulfate is also a food additive.[4]
References
- ↑ Handbook of Chemistry and Physics
- ↑ http://www.mindat.org/min-2584.html/ Mindat data
- ↑ Pink Book | Appendix B: Vaccine Excipient & Media Summary, Part 2
- ↑ Panera Bread › Menu & Nutrition › Nutrition Information Profile
Further reading
- Properties: UNIDO and International Fertilizer Development Center (1998), Fertilizer Manual, Kluwer Academic Publishers, ISBN 0-7923-5032-4.