Alzheimer's disease
| Alzheimer's disease Classification and external resources |
|
|
|
|
|---|---|
| Comparison of a normal aged brain (left) and an Alzheimer's patient's brain (right). Differential characteristics are pointed out. | |
| ICD-10 | G30., F00. |
| ICD-9 | 331.0, 290.1 |
| OMIM | 104300 |
| DiseasesDB | 490 |
| MedlinePlus | 000760 |
| eMedicine | neuro/13 |
| MeSH | D000544 |
Alzheimer's disease (AD), also called Alzheimer disease, Senile Dementia of the Alzheimer Type (SDAT) or simply Alzheimer's, is the most common form of dementia. This incurable, degenerative, and terminal disease was first described by German psychiatrist Alois Alzheimer in 1906. Generally it is diagnosed in people over 65 years of age,[1] although the less-prevalent early-onset Alzheimer's can occur much earlier. An estimated 26.6 million people worldwide had Alzheimer's in 2006; this number may quadruple by 2050.[2]
Although each sufferer experiences Alzheimer's in a unique way, there are many common symptoms.[3] The earliest observable symptoms are often mistakenly thought to be 'age-related' concerns, or manifestations of stress.[4] In the early stages, the most commonly recognised symptom is memory loss, such as difficulty in remembering recently learned facts. When a doctor or physician has been notified, and AD is suspected, the diagnosis is usually confirmed with behavioural assessments and cognitive tests, often followed by a brain scan if available.[5] As the disease advances, symptoms include confusion, irritability and aggression, mood swings, language breakdown, long-term memory loss, and the general withdrawal of the sufferer as their senses decline.[4][6] Gradually, bodily functions are lost, ultimately leading to death.[7] Individual prognosis is difficult to assess, as the duration of the disease varies. AD develops for an indeterminate period of time before becoming fully apparent, and it can progress undiagnosed for years. The mean life expectancy following diagnosis is approximately seven years.[8] Fewer than three percent of individuals live more than fourteen years after diagnosis.[9]
The cause and progression of Alzheimer's disease are not well understood. Research indicates that the disease is associated with plaques and tangles in the brain.[10] Currently-used treatments offer a small symptomatic benefit; no treatments to delay or halt the progression of the disease are as yet available. As of 2008, more than 500 clinical trials were investigating possible treatments for AD, but it is unknown if any of them will prove successful.[11] Many measures have been suggested for the prevention of Alzheimer's disease, but their value is unproven in slowing the course and reducing the severity of the disease. Mental stimulation, exercise, and a balanced diet are often recommended, as both a possible prevention and a sensible way of managing the disease.[12]
Because AD cannot be cured and is degenerative, management of patients is essential. The role of the main caregiver is often taken by the spouse or a close relative.[13] Alzheimer's disease is known for placing a great burden on caregivers; the pressures can be wide-ranging, involving social, psychological, physical, and economic elements of the caregiver's life.[14][15][16] In developed countries, AD is one of the most economically costly diseases to society.[17][18]
Contents |
Characteristics
The disease course is divided into four stages, with a progressive pattern of cognitive and functional impairment.
Predementia
The first symptoms are often mistaken as related to ageing or stress.[4] Detailed neuropsychological testing can reveal mild cognitive difficulties up to eight years before a person fulfills the clinical criteria for diagnosis of AD.[19] These early symptoms can affect the most complex daily living activities.[20] The most noticeable deficit is memory loss, which shows up as difficulty in remembering recently learned facts and inability to acquire new information.[21][22] Subtle problems with the executive functions of attentiveness, planning, flexibility, and abstract thinking, or impairments in semantic memory (memory of meanings, and concept relationships), can also be symptomatic of the early stages of AD.[23][24] Apathy can be observed at this stage, and remains the most persistent neuropsychiatric symptom throughout the course of the disease.[25][26][27] The preclinical stage of the disease has also been termed mild cognitive impairment,[28] but there is still debate on whether this term corresponds to a different diagnostic entity by itself or just a first step of the disease.[29]
Early dementia
In people with AD the increasing impairment of learning and memory eventually leads to a definitive diagnosis. In a small proportion of them, difficulties with language, executive functions, perception (agnosia), or execution of movements (apraxia) are more prominent than memory problems.[30] AD does not affect all memory capacities equally. Older memories of the person's life (episodic memory), facts learned (semantic memory), and implicit memory (the memory of the body on how to do things, such as using a fork to eat) are affected to a lesser degree than new facts or memories.[31][32] Language problems are mainly characterised by a shrinking vocabulary and decreased word fluency, which lead to a general impoverishment of oral and written language. In this stage, the person with Alzheimer's is usually capable of adequately communicating basic ideas.[33][34][35] While performing fine motor tasks such as writing, drawing or dressing, certain movement coordination and planning difficulties (apraxia) may be present, making sufferers appear clumsy.[36] As the disease progresses, people with AD can often continue to perform many tasks independently, but may need assistance or supervision with the most cognitively demanding activities.[30]
Moderate dementia
Progressive deterioration eventually hinders independence.[30] Speech difficulties become evident due to an inability to recall vocabulary, which leads to frequent incorrect word substitutions (paraphasias). Reading and writing skills are also progressively lost.[33][37] Complex motor sequences become less coordinated as time passes, reducing the ability to perform most normal daily living activities.[38] During this phase, memory problems worsen, and the person may fail to recognise close relatives.[39] Long-term memory, which was previously intact, becomes impaired,[40] and behavioural changes become more prevalent. Common neuropsychiatric manifestations are wandering, sundowning,[41] irritability and labile affect, leading to crying, outbursts of unpremeditated aggression, or resistance to caregiving. Approximately 30% of patients also develop illusionary misidentifications and other delusional symptoms.[42][25] Urinary incontinence can develop.[43] These symptoms create stress for relatives and caretakers, which can be reduced by moving the person from home care to other long-term care facilities.[30][44]
Advanced dementia
During this last stage of AD, the patient is completely dependent upon caregivers. Language is reduced to simple phrases or even single words, eventually leading to complete loss of speech.[33] Despite the loss of verbal language abilities, patients can often understand and return emotional signals.[45] Although aggressiveness can still be present, extreme apathy and exhaustion are much more common results.[30] Patients will ultimately not be able to perform even the most simple tasks without assistance. Muscle mass and mobility deteriorate to the point where they are bedridden,[46] and they lose the ability to feed themselves.[47] Finally comes death, usually caused directly by some external factor such as pressure ulcers or pneumonia, not by the disease itself.[48][49]
Causes
Three major competing hypotheses exist to explain the cause of the disease. The oldest, on which most currently available drug therapies are based, is the cholinergic hypothesis, which proposes that AD is caused by reduced synthesis of the neurotransmitter acetylcholine. The cholinergic hypothesis has not maintained widespread support, largely because medications intended to treat acetylcholine deficiency have not been very effective. Other cholinergic effects have also been proposed, for example, initiation of large-scale aggregation of amyloid,[50] leading to generalised neuroinflammation.[51]
In 1991, the amyloid hypothesis postulates that amyloid beta (Aβ) deposits are the fundamental cause of the disease.[52][53] It is a compelling theory because the gene for the amyloid beta precursor (APP) is located on chromosome 21, and people with trisomy 21 (Down Syndrome) who thus have an extra gene copy almost universally exhibit AD by 40 years of age.[54][55] Also APOE4, the major genetic risk factor for AD, leads to excess amyloid buildup in the brain before AD symptoms arise. Thus, Aβ deposition precedes clinical AD.[56] Further evidence comes from the finding that transgenic mice that express a mutant form of the human APP gene develop fibrillar amyloid plaques and Alzheimer's-like brain pathology.[57] An experimental vaccine was found to clear the amyloid plaques in early human trials, but it did not have any significant effect on dementia.[58]
Deposition of amyloid plaques does not correlate well with neuron loss.[59] This observation supports the tau hypothesis, the idea that tau protein abnormalities initiate the disease cascade.[53] In this model, hyperphosphorylated tau begins to pair with other threads of tau. Eventually, they form neurofibrillary tangles inside nerve cell bodies.[60] When this occurs, the microtubules disintegrate, collapsing the neuron's transport system. This may result first in malfunctions in biochemical communication between neurons and later in the death of the cells.[61]
Pathophysiology

Neuropathology
Alzheimer's disease is characterised by loss of neurons and synapses in the cerebral cortex and certain subcortical regions. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus.[51]
Both amyloid plaques and neurofibrillary tangles are clearly visible by microscopy in brains of those afflicted by AD.[10] Plaques are dense, mostly insoluble deposits of amyloid-beta protein and cellular material outside and around neurons. They continue to grow into insoluble twisted fibres within the nerve cell, often called tangles. Although many older individuals develop some plaques and tangles as a consequence of ageing, the brains of AD patients have a greater number of them in specific brain regions such as the temporal lobe.[62]
Biochemistry

Alzheimer's disease has been identified as a protein misfolding disease (proteopathy), caused by accumulation of abnormally folded A-beta and tau proteins in the brain.[63] Plaques are made up of small peptides, 39–43 amino acids in length, called beta-amyloid (also written as A-beta or Aβ). Beta-amyloid is a fragment from a larger protein called amyloid precursor protein (APP), a transmembrane protein that penetrates through the neuron's membrane. APP is critical to neuron growth, survival and post-injury repair.[64][65] In Alzheimer's disease, an unknown process causes APP to be divided into smaller fragments by enzymes through proteolysis.[66] One of these fragments gives rise to fibrils of beta-amyloid, which form clumps that deposit outside neurons in dense formations known as senile plaques.[10][67]
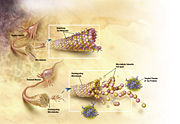
AD is also considered a tauopathy due to abnormal aggregation of the tau protein. Every neuron has a cytoskeleton, an internal support structure partly made up of structures called microtubules. These microtubules act like tracks, guiding nutrients and molecules from the body of the cell to the ends of the axon and back. A protein called tau stabilises the microtubules when phosphorylated, and is therefore called a microtubule-associated protein. In AD, tau undergoes chemical changes, becoming hyperphosphorylated; it then begins to pair with other threads, creating neurofibrillary tangles and disintegrating the neuron's transport system.[68]
Disease mechanism
Exactly how disturbances of production and aggregation of the beta amyloid peptide gives rise to the pathology of AD is not known.[69] The amyloid hypothesis traditionally points to the accumulation of beta amyloid peptides as the central event triggering neuron degeneration. Accumulation of aggregated amyloid fibrils, which are believed to be the toxic form of the protein responsible for disrupting the cell's calcium ion homeostasis, induces programmed cell death (apoptosis).[70] It is also known that Aβ selectively builds up in the mitochondria in the cells of Alzheimer's-affected brains, and it also inhibits certain enzyme functions and the utilisation of glucose by neurons.[71]
Various inflammatory processes and cytokines may also have a role in the pathology of Alzheimer's disease. Inflammation is a general marker of tissue damage in any disease, and may be either secondary to tissue damage in AD or a marker of an immunological response.[72]
Genetics
While the rare, early-onset form of Alzheimer's disease is mainly explained by mutations in three genes, the most common form has yet to be explained by a purely genetic model. The APOE gene is the strongest genetic risk factor for Alzheimer's discovered so far, but its presence is far from explaining all occurrences of the disease.[73]
Less than 10% of AD cases occurring before 60 years of age are due to autosomal dominant (familial) mutations, which therefore represent less than 0.01% of all cases.[73][74][75] These mutations have been discovered in three different genes: amyloid precursor protein (APP) and presenilins 1 and 2.[73] Most mutations in the APP and presenilin genes increase the production of a small protein called Abeta42, which is the main component of senile plaques.[76]
Most cases of Alzheimer's disease do not exhibit familial inheritance, but genes may act as risk factors. The best known genetic risk factor is the inheritance of the ε4 allele of the apolipoprotein E (APOE). This gene is implicated in up to 50% of late-onset sporadic Alzheimer's cases.[77] Geneticists agree that numerous other genes also act as risk factors or have protective effects that influence the development of late onset Alzheimer's disease.[73] Over 400 genes have been tested for association with late-onset sporadic AD.[78] One example is a variant of the reelin gene that may contribute to Alzheimer's risk in women.[79]
Diagnosis

Alzheimer's disease is usually diagnosed clinically from the patient history, collateral history from relatives, and clinical observations, based on the presence of characteristic neurological and neuropsychological features and the absence of alternative conditions.[80][81] Advanced medical imaging with computed tomography (CT) or magnetic resonance imaging (MRI), and with single photon emission computed tomography (SPECT) or positron emission tomography (PET) can be used to help exclude other cerebral pathology or subtypes of dementia.[82] Assessment of intellectual functioning including memory testing can further characterise the state of the disease.[4] Medical organisations have created diagnostic criteria to ease and standardise the diagnostic process for practicing physicians. Sometimes the diagnosis can be confirmed or made at post-mortem when brain material is available and can be examined histologically.[83]
Diagnostic criteria
The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (now known as the Alzheimer's Association) established the most commonly used diagnostic criteria for Alzheimer's disease.[84] These criteria require that the presence of cognitive impairment, and a suspected dementia syndrome, be confirmed by neuropsychological testing for a clinical diagnosis of possible or probable AD. A histopathologic confirmation including a microscopic examination of brain tissue is required for a definitive diagnosis. Good statistical reliability and validity have been shown between the diagnostic criteria and definitive histopathological confirmation.[85] Eight cognitive domains are most commonly impaired in AD—memory, language, perceptual skills, attention, constructive abilities, orientation, problem solving and functional abilities. These domains are equivalent to the NINCDS-ADRDA Alzheimer's Criteria as listed in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) published by the American Psychiatric Association.[86][87]
Diagnostic tools

Neuropsychological tests such as the mini-mental state examination (MMSE), are widely used to evaluate the cognitive impairments needed for diagnosis. More comprehensive test arrays are necessary for high reliability of results, particularly in the earliest stages of the disease.[88][89] Neurological examination in early AD will usually provide normal results, except for obvious cognitive impairment, which may not differ from standard dementia. Further neurological examinations are crucial in the differential diagnosis of AD and other diseases.[4] Interviews with family members are also utilised in the assessment of the disease. Caregivers can supply important information on the daily living abilities, as well as on the decrease, over time, of the person's mental function.[90] A caregiver's viewpoint is particularly important, since a person with AD is commonly unaware of his own deficits.[91] Many times, families also have difficulties in the detection of initial dementia symptoms and may not communicate accurate information to a physician.[92] Supplemental testing provides extra information on some features of the disease or is used to rule out other diagnoses. Blood tests can identify other causes for dementia than AD[4]—causes which may, in rare cases, be reversible.[93] Psychological tests for depression are employed, since depression can either be concurrent with AD or be the cause of cognitive impairment.[94][95]
When available as a diagnostic tool, SPECT and PET neuroimaging are used to confirm a diagnosis of Alzheimer's in conjunction with evaluations involving mental status examination.[96] The ability of SPECT to differentiate Alzheimer's disease from other possible causes in somebody already known to be suffering from dementia, appears to be superior to attempts to diagnose by mental testing and history.[97] A new technique known as PiB PET has been developed for directly and clearly imaging beta-amyloid deposits in vivo using a tracer that binds selectively to the Abeta deposits.[98] Another recent objective marker of the disease is the analysis of cerebrospinal fluid for amyloid beta or tau proteins.[99] Both advances have led to the proposal of new diagnostic criteria.[84][4]
Prevention

Global studies of measures to prevent or delay the onset of AD have often produced inconsistent results. At present, there appears to be no definitive evidence to support the belief that any particular measure is effective in preventing AD.[100] However, epidemiological studies have proposed relationships between certain modifiable factors, such as diet, cardiovascular risk, pharmaceutical products, or intellectual activities among others, and a population's likelihood of developing AD. Only further research, including clinical trials, will reveal whether, in fact, these factors can help to prevent AD.[101]
The components of a Mediterranean diet, which include fruit and vegetables, bread, wheat and other cereals, olive oil, fish, and red wine, may all individually or together reduce the risk and course of Alzheimer's disease.[102] Several vitamins such as B12, B3, C or folic acid have been found in some studies to be related to a reduced risk of AD[103] but other studies indicate that they do not have any significant effect on the onset or course of the disease and may have important secondary effects.[104] Curcumin from the curry spice turmeric has shown some effectiveness in preventing brain damage in mouse models.[105]
Although cardiovascular risk factors, such as hypercholesterolemia, hypertension, diabetes, and smoking, are associated with a higher risk of onset and course of AD,[106][107] statins, which are cholesterol lowering drugs, have not been effective in preventing or improving the course of the disease.[108][109] However long-term usage of non-steroidal anti-inflammatory drug (NSAIDs), is associated with a reduced likelihood of developing AD in some individuals.[110] Other pharmaceutical therapies such as female hormone replacement therapy are no longer thought to prevent dementia.[111][112] A 2007 systematic review concluded that there was inconsistent and unconvincing evidence that ginkgo has any positive effect on cognitive impairment,[113] and a recent study concludes that it has no effect in reducing the rate of AD incidence.[114]
Intellectual activities such as reading, playing board games, completing crossword puzzles, playing musical instruments, or regular social interaction may also delay the onset or reduce the severity of Alzheimer's disease.[115][116] Bilingualism is also related to a later onset of Alzheimer's.[117]
Some studies have shown an increased risk of developing AD with occupational exposure to magnetic fields,[118][119] intake of metals, particularly aluminium,[120][121] or exposure to solvents.[122] The quality of some of these studies has been criticised,[123] and other studies have concluded that there is no relationship between these environmental factors and the development of AD.[124][125][126][127]
Management
There is no cure for Alzheimer's disease; available treatments offer relatively small symptomatic benefit but remain palliative in nature. Current treatments can be divided into pharmaceutical, psychosocial and caregiving.
Pharmaceutical


Four medications are currently approved by regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) to treat the cognitive manifestations of AD: three are acetylcholinesterase inhibitors and the other is memantine, an NMDA receptor antagonist. No drug has an indication for delaying or halting the progression of the disease.
Reduction in the activity of the cholinergic neurons is a well-known feature of Alzheimer's disease.[128] Acetylcholinesterase inhibitors are employed to reduce the rate at which acetylcholine (ACh) is broken down, thereby increasing the concentration of ACh in the brain and combating the loss of ACh caused by the death of cholinergic neurons.[129] As of 2008, the cholinesterase inhibitors approved for the management of AD symptoms are donepezil (brand name Aricept),[130] galantamine (Razadyne),[131] and rivastigmine (branded as Exelon[132] and Exelon Patch[133]). There is evidence for the efficacy of these medications in mild to moderate Alzheimer’s disease,[134] and some evidence for their use in the advanced stage. Only donepezil is approved for treatment of advanced AD dementia.[135] The use of these drugs in mild cognitive impairment has not shown any effect in a delay of the onset of AD.[136] The most common side effects are nausea and vomiting, both of which are linked to cholinergic excess. These side effects arise in approximately ten to twenty percent of users and are mild to moderate in severity. Less common secondary effects include muscle cramps, decreased heart rate (bradycardia), decreased appetite and weight, and increased gastric acid production.[137]
Glutamate is a useful excitatory neurotransmitter of the nervous system, although excessive amounts in the brain can lead to cell death through a process called excitotoxicity which consists of the overstimulation of glutamate receptors. Excitotoxicity occurs not only in Alzheimer's disease, but also in other neurological diseases such as Parkinson's disease and multiple sclerosis.[138] Memantine (brand names Akatinol, Axura, Ebixa/Abixa, Memox and Namenda),[139] is a noncompetitive NMDA receptor antagonist first used as an anti-influenza agent. It acts on the glutamatergic system by blocking NMDA receptors and inhibiting their overstimulation by glutamate.[138] Memantine has been shown to be moderately efficacious in the treatment of moderate to severe Alzheimer’s disease. Its effects in the initial stages of AD are unknown.[140] Reported adverse events with memantine are infrequent and mild, including hallucinations, confusion, dizziness, headache and fatigue.[141] The combination of memantine and donepezil has been shown to be "of statistically significant but clinically marginal effectiveness".[142]
Antipsychotic drugs are modestly useful in reducing aggression and psychosis in Alzheimer's patients with behavioural problems, but are associated with serious adverse effects, such as cerebrovascular events, movement difficulties or cognitive decline, that do not permit their routine use.[143]
Psychosocial intervention
Psychosocial interventions are used as an adjunct to pharmaceutical treatment and can be classified within behaviour-, emotion-, cognition- or stimulation-oriented approaches. Research on efficacy is unavailable and rarely specific to AD, focusing instead on dementia in general.[144]
Behavioural interventions attempt to identify and reduce the antecedents and consequences of problem behaviours. This approach has not shown success in improving overall functioning,[145] but can help to reduce some specific problem behaviours, such as incontinence.[146] There is a lack of high quality data on the effectiveness of these techniques in other behaviour problems such as wandering.[147][148]
Emotion-oriented interventions include reminiscence therapy, validation therapy, supportive psychotherapy, sensory integration, also called snoezelen, and simulated presence therapy. Supportive psychotherapy has received little or no formal scientific study, but some clinicians find it useful in helping mildly impaired patients adjust to their illness.[144] Reminiscence therapy (RT) involves the discussion of past experiences individually or in group, many times with the aid of photographs, household items, music and sound recordings, or other familiar items from the past. Although there are few quality studies on the effectiveness of RT, it may be beneficial for cognition and mood.[149] Simulated presence therapy (SPT) is based on attachment theories and involves playing a recording with voices of the closest relatives of the person with Alzheimer's disease. There is preliminary evidence indicating that SPT may reduce anxiety and challenging behaviours.[150][151] Finally, validation therapy is based on acceptance of the reality and personal truth of another's experience, while sensory integration is based on exercises aimed to stimulate senses. There is little evidence to support the usefulness of these therapies.[152][153]
The aim of cognition-oriented treatments, which include reality orientation and cognitive retraining, is the reduction of cognitive deficits. Reality orientation consists in the presentation of information about time, place or person in order to ease the understanding of the person about its surroundings and his or her place in them. On the other hand cognitive retraining tries to improve impaired capacities by exercitation of mental abilities. Both have shown some efficacy improving cognitive capacities,[154][155] although in some studies these effects were transient and negative effects, such as frustration, have also been reported.[144]
Stimulation-oriented treatments include art, music and pet therapies, exercise, and any other kind of recreational activities. Stimulation has modest support for improving behaviour, mood, and, to a lesser extent, function. Nevertheless, as important as these effects are, the main support for the use of stimulation therapies is the improvement in the person's daily life routines.[144]
Caregiving
- Further information: Caregiving and dementia
Since Alzheimer's has no cure and it gradually renders people incapable of tending for their own needs, caregiving essentially is the treatment and must be carefully managed over the course of the disease.
During the early and moderate stages, modifications to the living environment and lifestyle can increase patient safety and reduce caretaker burden.[156][157] Examples of such modifications are the adherence to simplified routines, the placing of safety locks, the labelling of household items to cue the person with the disease or the use of modified daily life objects.[158][159][144] The patient may also become incapable of feeding themselves, so they require food in smaller pieces or pureed.[160] When swallowing difficulties arise, the use of feeding tubes may be required. In such cases, the medical efficacy and ethics of continuing feeding is an important consideration of the caregivers and family members.[161][162] The use of physical restraints is rarely indicated in any stage of the disease, although there are situations when they are necessary to prevent harm to the person with AD or their caregivers.[144]
As the disease progresses, different medical issues can appear, such as oral and dental disease, pressure ulcers, malnutrition, hygiene problems, or respiratory, skin, or eye infections. Careful management can prevent them, while professional treatment is needed when they do arise.[163][49] During the final stages of the disease, treatment is centred on relieving discomfort until death.[164]
Prognosis
The early stages of Alzheimer's disease are difficult to diagnose. A definitive diagnosis is usually made once cognitive impairment compromises daily living activities, although the person may still be living independently. He will progress from mild cognitive problems, such as memory loss through increasing stages of cognitive and non-cognitive disturbances, eliminating any possibility of independent living.[30]
Life expectancy of the population with the disease is reduced.[8][165][166] The mean life expectancy following diagnosis is approximately seven years.[8] Fewer than 3% of patients live more than fourteen years.[9] Disease features significantly associated with reduced survival are an increased severity of cognitive impairment, decreased functional level, history of falls, and disturbances in the neurological examination. Other coincident diseases such as heart problems, diabetes or history of alcohol abuse are also related with shortened survival.[167][165][168] While the earlier the age at onset the higher the total survival years, life expectancy is particularly reduced when compared to the healthy population among those who are younger.[166] Men have a less favourable survival prognosis than women.[9][169]
The disease is the underlying cause of death in 70% of all cases.[8] Pneumonia and dehydration are the most frequent immediate causes of death, while cancer is a less frequent cause of death than in the general population.[8][169]
Epidemiology
| Age | Incidence (new affected) per thousand person–years |
|---|---|
| 65–69 | 3 |
| 70–74 | 6 |
| 75–79 | 9 |
| 80–84 | 23 |
| 85–89 | 40 |
| 90– | 69 |
Two main measures are used in epidemiological studies: incidence and prevalence. Incidence is the number of new cases per unit of person–time at risk (usually number of new cases per thousand person–years); while prevalence is the total number of cases of the disease in the population at a given time.
Regarding incidence, cohort longitudinal studies (studies where a disease-free population is followed over the years) provide rates between 10–15 per thousand person–years for all dementias and 5–8 for AD,[170][171] which means that half of new dementia cases each year are AD. Advancing age is a primary risk factor for the disease and incidence rates are not equal for all ages: every five years after the age of 65, the risk of acquiring the disease approximately doubles, increasing from 3 to as much as 69 per thousand person years.[170][171] There are also sex differences in the incidence rates, women having a higher risk of developing AD particularly in the population older than 85.[171][172]
Prevalence of AD in populations is dependent upon different factors including incidence and survival. Since the incidence of AD increases with age, it is particularly important to include the mean age of the population of interest. In the United States, Alzheimer prevalence was estimated to be 1.6% in the year 2000 both overall and in the 65–74 age group, with the rate increasing to 19% in the 75–84 group and to 42% in the greater than 84 group.[173] Prevalence rates in less developed regions are lower.[174] The World Health Organization estimated that in 2005, 0.379% of people worldwide had dementia, and that the prevalence would increase to 0.441% in 2015 and to 0.556% in 2030.[175] Other studies have reached similar conclusions.[174] Another study estimated that in 2006, 0.40% of the world population (range 0.17–0.89%; absolute number 26.6 million, range 11.4–59.4 million) were afflicted by AD, and that the prevalence rate would triple and the absolute number would quadruple by the year 2050.[2]
History

The ancient Greek and Roman philosophers and physicians associated old age with increasing dementia.[176] It was not until 1901 that German psychiatrist Alois Alzheimer identified the first case of what became known as Alzheimer's disease in a fifty-year-old woman he called Auguste D. Alzheimer followed her until she died in 1906, when he first reported the case publicly.[177] During the next five years, eleven similar cases were reported in the medical literature, some of them already using the term Alzheimer's disease.[176] The disease was first described as a distinctive disease by Emil Kraepelin, who included Alzheimer’s disease, also named presenile dementia by Kraepelin, as a subtype of senile dementia in the eighth edition of his Textbook of Psychiatry, published in 1910.[178]
For most of the twentieth century, the diagnosis of Alzheimer's disease was reserved for individuals between the ages of 45 and 65 who developed symptoms of dementia. The terminology changed after 1977 when a conference on AD concluded that the clinical and pathological manifestations of presenile and senile dementia were almost identical, although the authors also added that this did not rule out the possibility of different aetiologies.[179] This eventually led to the diagnosis of Alzheimer's disease independently of age.[180] The term senile dementia of the Alzheimer type (SDAT) was used for a time to describe the condition in those over 65, with classical Alzheimer's disease being used for those younger. Eventually, the term Alzheimer's disease was formally adopted in medical nomenclature to describe individuals of all ages with a characteristic common symptom pattern, disease course, and neuropathology.[181]
Society and culture
Social costs
Dementia, and specifically Alzheimer's disease, may be among the most costly diseases for society in the developed countries,[17][18] while their cost in developing countries such as Argentina,[182] or developed South Korea,[183] is also high and rising. These costs will probably increase with the ageing of society, becoming an important social problem. AD associated costs include direct medical costs such as nursing home care, direct nonmedical costs such as in-home day care, and indirect costs such as lost productivity of both patient and caregiver.[18] Numbers vary between studies but dementia costs worldwide have been calculated around $160 billion,[184] while costs of Alzheimer in the United States may be $100 billion each year.[18]
The greatest origin of costs for society is the long-term care by health care professionals and particularly institutionalisation, which corresponds to 2/3 of the total costs for society.[17] The cost of living at home is also very high,[17] especially when informal costs for the family, such as caregiving time and caregiver's lost earnings, are taken into account.[185]
Costs increase with dementia severity and the presence of behavioural disturbances,[186] and are related to the increased caregiving time required for the provision of physical care.[185] Therefore any treatment that slows cognitive decline, delays institutionalisation or reduces caregivers' hours will have economic benefits. Economic evaluations of current treatments have shown positive results.[18]
Caregiving burden
- Further information: Caregiving and dementia
The role of the main caregiver is often taken by the spouse or a close relative.[187] Alzheimer's disease is known for placing a great burden on caregivers which includes social, psychological, physical or economic aspects.[14][15][16] Home care is usually preferred by patients and families.[188] This option also delays or eliminates the need for more professional and costly levels of care.[189][188] Nevertheless two-thirds of nursing home residents have dementias.[144]
Dementia caregivers are subject to high rates of physical and mental disorders.[190] Factors associated with greater psychosocial problems of the primary caregivers include having an affected person at home, the carer being a spouse, demanding behaviours of the cared person such as depression, behavioural disturbances, hallucinations, sleep problems or walking disruptions and social isolation.[191][192] Regarding economic problems, family caregivers often give up time from work to spend 47 hours per week on average with the person with AD, while the costs of caring for them are high. Direct and indirect costs of caring for an Alzheimer's patient average between $18,000 and $77,500 per year in the United States, depending on the study.[193][185]
Cognitive behavioural therapy and the teaching of coping strategies either individually or in group have demonstrated their efficacy in improving caregivers' psychological health.[14][194]
Notable cases
- Further information: Alzheimer's in the media

As Alzheimer's disease is highly prevalent, many notable people have developed it. Well-known examples are former United States President Ronald Reagan and Irish writer Iris Murdoch, both of whom were the subjects of scientific articles examining how their cognitive capacities deteriorated with the disease.[195][196] Other notable cases include the retired footballer Ferenc Puskas,[197] the former Prime Ministers Harold Wilson (Great Britain) and Adolfo Suárez (Spain),[198][199] the actress Rita Hayworth,[200] the actor Charlton Heston,[201] and the novelist Terry Pratchett.[202]
AD has also been portrayed in films such as: Iris (2001),[203] based on John Bayley's memoir of his wife Iris Murdoch;[204] The Notebook (2004),[205] based on Nicholas Sparks' 1996 novel of the same name;[206] Thanmathra (2005);[207] Memories of Tomorrow (Ashita no Kioku) (2006),[208] based on Hiroshi Ogiwara's novel of the same name;[209] and Away from Her (2006), based on Alice Munro's short story "The Bear Came over the Mountain".[210] Documentaries on Alzheimer's disease include Malcolm and Barbara: A Love Story (1999) and Malcolm and Barbara: Love’s Farewell (2007), both featuring Malcolm Pointon.[211]
Research directions
As of 2008, the safety and efficacy of more than 400 pharmaceutical treatments are being investigated in clinical trials worldwide, and approximately one-fourth of these compounds are in Phase III trials, which is the last step prior to review by regulatory agencies.[212]
One area of clinical research is focused on treating the underlying disease pathology. Reduction of amyloid beta levels is a common target of compounds under investigation. Immunotherapy or vaccination for the amyloid protein is one treatment modality under study. Unlike preventative vaccination, the putative therapy would be used to treat people already diagnosed. It is based upon the concept of training the immune system to recognise, attack, and reverse deposition of amyloid, thereby altering the course of the disease.[213] An example of such a vaccine under investigation was ACC-001,[214][215] although the trials were suspended in 2008.[216] Another similar agent is bapineuzumab, an antibody designed as identical to the naturally-induced anti-amyloid antibody. [217] Other approaches are neuroprotective agents, such as AL-108,[218] and metal-protein interaction attenuation agents, such as PBT2.[219] A TNFα receptor fusion protein, etanercept has showed encouraging results.[220]
In 2008, two separate clinical trials showed positive results in modifying the course of disease in mild to moderate AD with methylthioninium chloride (trade name rember), a drug that inhibits tau aggregation,[221][222] and dimebon, an antihistamine.[223]
References
- ↑ Brookmeyer R, Gray S, Kawas C (September 1998). "Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset". Am J Public Health 88 (9): 1337–42. PMID 9736873.
- ↑ 2.0 2.1 2006 prevalence estimate:
- Brookmeyer R, Johnson E, Ziegler-Graham K, MH Arrighi (July 2007). "Forecasting the global burden of Alzheimer’s disease". Alzheimer's and Dementia 3 (3): 186–91. doi:. http://works.bepress.com/cgi/viewcontent.cgi?article=1022&context=rbrookmeyer. Retrieved on 2008-06-18.
- . "World population prospects: the 2006 revision, highlights" (PDF). Working Paper No. ESA/P/WP.202. Population Division, Department of Economic and Social Affairs, United Nations. Retrieved on 2008-08-27.
- ↑ "What is Alzheimer’s disease?". Alzheimers.org.uk (August 2007). Retrieved on 2008-02-21.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Waldemar G, Dubois B, Emre M, et al (January 2007). "Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline". Eur. J. Neurol. 14 (1): e1–26. doi:. PMID 17222085.
- ↑ "Alzheimer's diagnosis of AD". Alzheimer's Research Trust. Retrieved on 2008-02-29.
- ↑ Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP (2005). "A 10-item smell identification scale related to risk for Alzheimer's disease". Ann. Neurol. 58 (1): 155–160. doi:. PMID 15984022.
- ↑ "Understanding stages and symptoms of Alzheimer's disease". National Institute on Aging (2007-10-26). Retrieved on 2008-02-21.
- ↑ 8.0 8.1 8.2 8.3 8.4 Mölsä PK, Marttila RJ, Rinne UK (August 1986). "Survival and cause of death in Alzheimer's disease and multi-infarct dementia". Acta Neurol. Scand. 74 (2): 103–7. PMID 3776457.
- ↑ 9.0 9.1 9.2 Mölsä PK, Marttila RJ, Rinne UK (March 1995). "Long-term survival and predictors of mortality in Alzheimer's disease and multi-infarct dementia". Acta Neurol. Scand. 91 (3): 159–64. PMID 7793228.
- ↑ 10.0 10.1 10.2 Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (June 2004). "The importance of neuritic plaques and tangles to the development and evolution of AD". Neurology 62 (11): 1984–9. PMID 15184601.
- ↑ "Alzheimer's Disease Clinical Trials". US National Institutes of Health. Retrieved on 2008-08-18.
- ↑ "Can Alzheimer's disease be prevented" (pdf). National Institute on Aging (2006-08-29). Retrieved on 2008-02-29.
- ↑ "The MetLife study of Alzheimer’s disease: The caregiving experience" (PDF). MetLife Mature Market Institute (August 2006). Retrieved on 2008-02-12.
- ↑ 14.0 14.1 14.2 Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J (2007). "Systematic review of information and support interventions for caregivers of people with dementia". BMC Geriatr 7: 18. doi:. PMID 17662119.
- ↑ 15.0 15.1 Schneider J, Murray J, Banerjee S, Mann A (August 1999). "EUROCARE: a cross-national study of co-resident spouse carers for people with Alzheimer's disease: I—Factors associated with carer burden". International Journal of Geriatric Psychiatry 14 (8): 651–661. doi:. PMID 10489656.
- ↑ 16.0 16.1 Murray J, Schneider J, Banerjee S, Mann A (August 1999). "EUROCARE: a cross-national study of co-resident spouse carers for people with Alzheimer's disease: II--A qualitative analysis of the experience of caregiving". International Journal of Geriatric Psychiatry 14 (8): 662–667. doi:. PMID 10489657.
- ↑ 17.0 17.1 17.2 17.3 Bonin-Guillaume S, Zekry D, Giacobini E, Gold G, Michel JP (January 2005). "Impact économique de la démence (English: The economical impact of dementia)" (in French). Presse Med 34 (1): 35–41. ISSN 0755-4982. PMID 15685097.
- ↑ 18.0 18.1 18.2 18.3 18.4 Meek PD, McKeithan K, Schumock GT (1998). "Economic considerations in Alzheimer's disease". Pharmacotherapy 18 (2 Pt 2): 68–73; discussion 79–82. PMID 9543467.
- ↑ Preclinical:
- Linn RT, Wolf PA, Bachman DL, et al (May 1995). "The 'preclinical phase' of probable Alzheimer's disease. A 13-year prospective study of the Framingham cohort". Arch. Neurol. 52 (5): 485–90. PMID 7733843.
- Saxton J, Lopez OL, Ratcliff G, et al (December 2004). "Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset". Neurology 63 (12): 2341–7. PMID 15623697.
- Twamley EW, Ropacki SA, Bondi MW (September 2006). "Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease". J Int Neuropsychol Soc 12 (5): 707–35. doi:. PMID 16961952.
- ↑ Perneczky R, Pohl C, Sorg C, Hartmann J, Komossa K, Alexopoulos P, Wagenpfeil S, Kurz A (2006). "Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues". Age Ageing 35 (3): 240–245. doi:. PMID 16513677.
- ↑ Arnáiz E, Almkvist O (2003). "Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease". Acta Neurol. Scand., Suppl. 179: 34–41. doi:. PMID 12603249.
- ↑ Kazui H, Matsuda A, Hirono N, et al (2005). "Everyday memory impairment of patients with mild cognitive impairment". Dement Geriatr Cogn Disord 19 (5–6): 331–7. doi:. PMID 15785034. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=DEM20050195_6331. Retrieved on 2008-06-12.
- ↑ Rapp MA, Reischies FM (2005). "Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE)". American Journal of Geriatric Psychiatry 13 (2): 134–141. doi:. PMID 15703322.
- ↑ Spaan PE, Raaijmakers JG, Jonker C (2003). "Alzheimer's disease versus normal ageing: a review of the efficiency of clinical and experimental memory measures". Journal of Clinical Experimental Neuropsychology 25 (2): 216–233. PMID 12754679.
- ↑ 25.0 25.1 Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP (2005). "A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer's disease". American Journal of Geriatric Psychiatry 13 (6): 460–468. doi:. PMID 15956265.
- ↑ Robert PH, Berr C, Volteau M, Bertogliati C, Benoit M, Sarazin M, Legrain S, Dubois B (2006). "Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer's disease: a one-year follow-up study". Clin Neurol Neurosurg 108 (8): 733–736. doi:. PMID 16567037.
- ↑ Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L (2007). "Predictors of progression from mild cognitive impairment to Alzheimer disease". Neurology 68 (19): 1596–1602. doi:. PMID 17485646.
- ↑ Small BJ, Gagnon E, Robinson B (April 2007). "Early identification of cognitive deficits: preclinical Alzheimer's disease and mild cognitive impairment". Geriatrics 62 (4): 19–23. PMID 17408315.
- ↑ Petersen RC (February 2007). "The current status of mild cognitive impairment—what do we tell our patients?". Nat Clin Pract Neurol 3 (2): 60–1. doi:. PMID 17279076.
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 Förstl H, Kurz A (1999). "Clinical features of Alzheimer's disease". European Archives of Psychiatry and Clinical Neuroscience 249 (6): 288–290. PMID 10653284.
- ↑ Carlesimo GA, Oscar-Berman M (June 1992). "Memory deficits in Alzheimer's patients: a comprehensive review". Neuropsychol Rev 3 (2): 119–69. PMID 1300219.
- ↑ Jelicic M, Bonebakker AE, Bonke B (1995). "Implicit memory performance of patients with Alzheimer's disease: a brief review". International Psychogeriatrics 7 (3): 385–392. doi:. PMID 8821346.
- ↑ 33.0 33.1 33.2 Frank EM (September 1994). "Effect of Alzheimer's disease on communication function". J S C Med Assoc 90 (9): 417–23. PMID 7967534.
- ↑ Becker JT, Overman AA (2002). "[The semantic memory deficit in Alzheimer's disease]" (in Spanish; Castilian). Rev Neurol 35 (8): 777–83. PMID 12402233.
- ↑ Hodges JR, Patterson K (April 1995). "Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications". Neuropsychologia 33 (4): 441–59. PMID 7617154.
- ↑ Benke T (December 1993). "Two forms of apraxia in Alzheimer's disease". Cortex 29 (4): 715–25. PMID 8124945.
- ↑ Forbes KE, Shanks MF, Venneri A (March 2004). "The evolution of dysgraphia in Alzheimer's disease". Brain Res. Bull. 63 (1): 19–24. doi:. PMID 15121235.
- ↑ Galasko D, Schmitt F, Thomas R, Jin S, Bennett D (2005). "Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease". Journal of the International Neuropsychology Society 11 (4): 446–453. PMID 16209425.
- ↑ Galasko D, Schmitt F, Thomas R, Jin S, Bennett D (July 2005). "Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease". J Int Neuropsychol Soc 11 (4): 446–53. PMID 16209425.
- ↑ Sartori G, Snitz BE, Sorcinelli L, Daum I (September 2004). "Remote memory in advanced Alzheimer's disease". Arch Clin Neuropsychol 19 (6): 779–89. doi:. PMID 15288331.
- ↑ Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A (May 2001). "Sundowning and circadian rhythms in Alzheimer's disease". Am J Psychiatry 158 (5): 704–11. PMID 11329390. http://ajp.psychiatryonline.org/cgi/content/full/158/5/704. Retrieved on 2008-08-27.
- ↑ Neuropsychiatric symptoms:
- Scarmeas N, Brandt J, Blacker D, et al (December 2007). "Disruptive behavior as a predictor in Alzheimer disease". Arch. Neurol. 64 (12): 1755–61. doi:. PMID 18071039.
- Tatsch MF, Bottino CM, Azevedo D, et al (May 2006). "Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity". Am J Geriatr Psychiatry 14 (5): 438–45. doi:. PMID 16670248.
- Volicer L, Bass EA, Luther SL (October 2007). "Agitation and resistiveness to care are two separate behavioral syndromes of dementia". J Am Med Dir Assoc 8 (8): 527–32. doi:. PMID 17931577.
- ↑ Honig LS, Mayeux R (June 2001). "Natural history of Alzheimer's disease". Aging (Milano) 13 (3): 171–82. PMID 11442300.
- ↑ Gold DP, Reis MF, Markiewicz D, Andres D (January 1995). "When home caregiving ends: a longitudinal study of outcomes for caregivers of relatives with dementia". J Am Geriatr Soc 43 (1): 10–6. PMID 7806732.
- ↑ Bär M, Kruse A, Re S (December 2003). "[Situations of emotional significance in residents suffering from dementia]" (in German). Z Gerontol Geriatr 36 (6): 454–62. doi:. PMID 14685735.
- ↑ Souren LE, Franssen EH, Reisberg B (June 1995). "Contractures and loss of function in patients with Alzheimer's disease". J Am Geriatr Soc 43 (6): 650–5. PMID 7775724.
- ↑ Berkhout AM, Cools HJ, van Houwelingen HC (September 1998). "The relationship between difficulties in feeding oneself and loss of weight in nursing-home patients with dementia". Age Ageing 27 (5): 637–41. PMID 12675103.
- ↑ Wada H, Nakajoh K, Satoh-Nakagawa T, et al (2001). "Risk factors of aspiration pneumonia in Alzheimer's disease patients". Gerontology 47 (5): 271–6. PMID 11490146.
- ↑ 49.0 49.1 Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R (July 1999). "Predictors of mortality in patients with Alzheimer's disease living in nursing homes". J. Neurol. Neurosurg. Psychiatr. 67 (1): 59–65. PMID 10369823.
- ↑ Shen ZX (2004). "Brain cholinesterases: II. The molecular and cellular basis of Alzheimer's disease". Med. Hypotheses 63 (2): 308–21. doi:. PMID 15236795.
- ↑ 51.0 51.1 Wenk GL (2003). "Neuropathologic changes in Alzheimer's disease". J Clin Psychiatry 64 Suppl 9: 7–10. PMID 12934968.
- ↑ Hardy J, Allsop D (October 1991). "Amyloid deposition as the central event in the aetiology of Alzheimer's disease". Trends Pharmacol. Sci. 12 (10): 383–88. PMID 1763432.
- ↑ 53.0 53.1 Mudher A, Lovestone S (January 2002). "Alzheimer's disease-do tauists and baptists finally shake hands?". Trends Neurosci. 25 (1): 22–26. PMID 11801334.
- ↑ Nistor M, Don M, Parekh M, et al (October 2007). "Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain". Neurobiol. Aging 28 (10): 1493–1506. doi:. PMID 16904243.
- ↑ Lott IT, Head E (March 2005). "Alzheimer disease and Down syndrome: factors in pathogenesis". Neurobiol. Aging 26 (3): 383–89. doi:. PMID 15639317.
- ↑ Polvikoski T, Sulkava R, Haltia M, et al (November 1995). "Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein". N. Engl. J. Med. 333 (19): 1242–47. PMID 7566000.
- ↑ Transgenic mice:
- Games D, Adams D, Alessandrini R, et al (February 1995). "Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein". Nature 373 (6514): 523–27. doi:. PMID 7845465.
- Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D (September 1996). "Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease". J. Neurosci. 16 (18): 5795–811. PMID 8795633.
- Hsiao K, Chapman P, Nilsen S, et al (October 1996). "Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice". Science (journal) 274 (5284): 99–102. PMID 8810256.
- ↑ Holmes C, Boche D, Wilkinson D, et al (July 2008). "Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial". Lancet 372 (9634): 216–23. doi:. PMID 18640458.
- ↑ Schmitz C, Rutten BP, Pielen A, et al (April 2004). "Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer's disease". Am. J. Pathol. 164 (4): 1495–1502. PMID 15039236.
- ↑ Goedert M, Spillantini MG, Crowther RA (July 1991). "Tau proteins and neurofibrillary degeneration". Brain Pathol. 1 (4): 279–86. PMID 1669718.
- ↑ Chun W, Johnson GV (2007). "The role of tau phosphorylation and cleavage in neuronal cell death". Front. Biosci. 12: 733–56. PMID 17127334.
- ↑ Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994). "Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital". Cereb. Cortex 4 (2): 138–50. PMID 8038565.
- ↑ Hashimoto M, Rockenstein E, Crews L, Masliah E (2003). "Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases". Neuromolecular Med. 4 (1–2): 21–36. doi:. PMID 14528050.
- ↑ Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (July 2006). "Synapse formation and function is modulated by the amyloid precursor protein". J. Neurosci. 26 (27): 7212–21. doi:. PMID 16822978.
- ↑ Turner PR, O'Connor K, Tate WP, Abraham WC (May 2003). "Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory". Prog. Neurobiol. 70 (1): 1–32. PMID 12927332.
- ↑ Hooper NM (April 2005). "Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein". Biochem. Soc. Trans. 33 (Pt 2): 335–8. doi:. PMID 15787600.
- ↑ Ohnishi S, Takano K (March 2004). "Amyloid fibrils from the viewpoint of protein folding". Cell. Mol. Life Sci. 61 (5): 511–24. doi:. PMID 15004691.
- ↑ Hernández F, Avila J (September 2007). "Tauopathies". Cell. Mol. Life Sci. 64 (17): 2219–33. doi:. PMID 17604998.
- ↑ Van Broeck B, Van Broeckhoven C, Kumar-Singh S (2007). "Current insights into molecular mechanisms of Alzheimer disease and their implications for therapeutic approaches". Neurodegener Dis 4 (5): 349–65. doi:. PMID 17622778.
- ↑ Yankner BA, Duffy LK, Kirschner DA (October 1990). "Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides". Science (journal) 250 (4978): 279–82. PMID 2218531.
- ↑ Chen X, Yan SD (December 2006). "Mitochondrial Abeta: a potential cause of metabolic dysfunction in Alzheimer's disease". IUBMB Life 58 (12): 686–94. doi:. PMID 17424907.
- ↑ Greig NH, Mattson MP, Perry T, et al (December 2004). "New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists". Ann. N. Y. Acad. Sci. 1035: 290–315. doi:. PMID 15681814.
- ↑ 73.0 73.1 73.2 73.3 Waring SC, Rosenberg RN (March 2008). "Genome-wide association studies in Alzheimer disease". Arch. Neurol. 65 (3): 329–34. doi:. PMID 18332245.
- ↑ Hoenicka J (2006 March 1–15). "Genes in Alzheimer's disease". Rev Neurol 42 (5): 302–05. PMID 16538594.
- ↑ Campion D, Dumanchin C, Hannequin D, et al (September 1999). "Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum". Am. J. Hum. Genet. 65 (3): 664–70. doi:. PMID 10441572.
- ↑ Selkoe DJ (June 1999). "Translating cell biology into therapeutic advances in Alzheimer's disease". Nature 399 (6738 Suppl): A23–31. PMID 10392577.
- ↑ Strittmatter WJ, Saunders AM, Schmechel D, et al (March 1993). "Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease". Proc. Natl. Acad. Sci. USA 90 (5): 1977–81. PMID 8446617.
- ↑ Waring SC, Rosenberg RN (March 2008). "Genome-wide association studies in Alzheimer disease". Arch. Neurol. 65 (3): 329–34. doi:. PMID 18332245.
- ↑ Seripa D, Matera MG, Franceschi M, et al (July 2008). "The RELN locus in Alzheimer's disease". J Alzheimers Dis. 14 (3): 335–44. PMID 18599960.
- ↑ Mendez MF (2006). "The accurate diagnosis of early-onset dementia". International Journal of Psychiatry Medicine 36 (4): 401–412. PMID 17407994.
- ↑ Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J (November 2006). "Therapeutic approaches to Alzheimer's disease". Brain 129 (Pt 11): 2840–55. doi:. PMID 17018549.
- ↑ "Dementia: Quick reference guide" (PDF). London: (UK) National Institute for Health and Clinical Excellence (November 2006). Retrieved on 2008-02-22.
- ↑ McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (July 1984). "Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease". Neurology 34 (7): 939–44. PMID 6610841.
- ↑ 84.0 84.1 Dubois B, Feldman HH, Jacova C, et al (August 2007). "Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria". Lancet Neurol 6 (8): 734–46. doi:. PMID 17616482.
- ↑ Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF (December 1994). "Reliability and validity of NINCDS-ADRDA criteria for Alzheimer's disease. The National Institute of Mental Health Genetics Initiative". Arch. Neurol. 51 (12): 1198–204. PMID 7986174.
- ↑ American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th Edition Text Revision ed.). Washington DC: American Psychiatric Association. ISBN 0890420254.
- ↑ Ito N (May 1996). "[Clinical aspects of dementia]" (in Japanese). Hokkaido Igaku Zasshi 71 (3): 315–20. PMID 8752526.
- ↑ Tombaugh TN, McIntyre NJ (September 1992). "The mini-mental state examination: a comprehensive review". J Am Geriatr Soc 40 (9): 922–35. PMID 1512391.
- ↑ Pasquier F (January 1999). "Early diagnosis of dementia: neuropsychology". J. Neurol. 246 (1): 6–15. PMID 9987708.
- ↑ Harvey PD, Moriarty PJ, Kleinman L, et al (2005). "The validation of a caregiver assessment of dementia: the Dementia Severity Scale". Alzheimer Dis Assoc Disord 19 (4): 186–94. PMID 16327345.
- ↑ Antoine C, Antoine P, Guermonprez P, Frigard B (2004). "[Awareness of deficits and anosognosia in Alzheimer's disease.]" (in French). Encephale 30 (6): 570–7. PMID 15738860.
- ↑ Cruz VT, Pais J, Teixeira A, Nunes B (2004). "[The initial symptoms of Alzheimer disease: caregiver perception]" (in Portuguese). Acta Med Port 17 (6): 435–44. PMID 16197855.
- ↑ Clarfield AM (October 2003). "The decreasing prevalence of reversible dementias: an updated meta-analysis". Arch. Intern. Med. 163 (18): 2219–29. doi:. PMID 14557220.
- ↑ Geldmacher DS, Whitehouse PJ (May 1997). "Differential diagnosis of Alzheimer's disease". Neurology 48 (5 Suppl 6): S2–9. PMID 9153154.
- ↑ Potter GG, Steffens DC (May 2007). "Contribution of depression to cognitive impairment and dementia in older adults". Neurologist 13 (3): 105–17. doi:. PMID 17495754.
- ↑ Bonte FJ, Harris TS, Hynan LS, Bigio EH, White CL (July 2006). "Tc-99m HMPAO SPECT in the differential diagnosis of the dementias with histopathologic confirmation". Clin Nucl Med 31 (7): 376–8. doi:. PMID 16785801.
- ↑ Dougall NJ, Bruggink S, Ebmeier KP (2004). "Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia". Am J Geriatr Psychiatry 12 (6): 554–70. doi:. PMID 15545324.
- ↑ PiB PET:
- Kemppainen NM, Aalto S, Karrasch M, et al (January 2008). "Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease". Ann. Neurol. 63 (1): 112–8. doi:. PMID 18023012.
- Ikonomovic MD, Klunk WE, Abrahamson EE, et al (June 2008). "Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease". Brain 131 (Pt 6): 1630–45. doi:. PMID 18339640.
- Jack CR, Lowe VJ, Senjem ML, et al (March 2008). "11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment". Brain 131 (Pt 3): 665–80. doi:. PMID 18263627.
- ↑ Marksteiner J, Hinterhuber H, Humpel C (June 2007). "Cerebrospinal fluid biomarkers for diagnosis of Alzheimer's disease: beta-amyloid(1-42), tau, phospho-tau-181 and total protein". Drugs Today 43 (6): 423–31. doi:. PMID 17612711.
- ↑ Prevention recommendations not supported:
- Kawas CH (2006). "Medications and diet: protective factors for AD?". Alzheimer Dis Assoc Disord 20 (3 Suppl 2): S89–96. PMID 16917203.
- Luchsinger JA, Mayeux R (2004). "Dietary factors and Alzheimer's disease". Lancet Neurol 3 (10): 579–87. doi:. PMID 15380154.
- Luchsinger JA, Noble JM, Scarmeas N (2007). "Diet and Alzheimer's disease". Curr Neurol Neurosci Rep 7 (5): 366–72. doi:. PMID 17764625.
- ↑ Szekely CA, Breitner JC, Zandi PP (2007). "Prevention of Alzheimer's disease". Int Rev Psychiatry 19 (6): 693–706. doi:. PMID 18092245.
- ↑ Mediterranean diet:
- Scarmeas N, Stern Y, Mayeux R, Luchsinger JA (2006). "Mediterranean diet, Alzheimer disease, and vascular mediation". Arch. Neurol. 63 (12): 1709–1717. doi:. PMID 17030648.
- Scarmeas N, Luchsinger JA, Mayeux R, Stern Y (2007). "Mediterranean diet and Alzheimer disease mortality". Neurology 69 (11): 1084–93. doi:. PMID 17846408.
- Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alpérovitch A (2007). "Dietary patterns and risk of dementia: the Three-City cohort study". Neurology 69 (20): 1921–1930. doi:. PMID 17998483.
- Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB (2006). "Fruit and vegetable juices and Alzheimer's disease: the Kame Project". American Journal of Medicine 119 (9): 751–759. doi:. PMID 16945610.
- Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Müller-Spahn F (2003). "Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity". Gerontology 49 (6): 380–383. doi:. PMID 14624067.
- ↑ Vitamins prevent:
- Morris MC, Schneider JA, Tangney CC (2006). "Thoughts on B-vitamins and dementia". J. Alzheimers Dis. 9 (4): 429–33. PMID 16917152.
- Landmark K (2006). "[Could intake of vitamins C and E inhibit development of Alzheimer dementia?]" (in Norwegian). Tidsskr. Nor. Laegeforen. 126 (2): 159–61. PMID 16415937.
- Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R (2007). "Relation of higher folate intake to lower risk of Alzheimer disease in the elderly". Arch. Neurol. 64 (1): 86–92. doi:. PMID 17210813.
- Morris MC, Evans DA, Bienias JL, et al (August 2004). "Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline". J. Neurol. Neurosurg. Psychiatr. 75 (8): 1093–9. doi:. PMID 15258207.
- ↑ Vitamins do not prevent:
- Morris MC, Evans DA, Schneider JA, Tangney CC, Bienias JL, Aggarwal NT (2006). "Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer's disease". J. Alzheimers Dis. 9 (4): 435–43. PMID 16917153.
- Malouf M, Grimley EJ, Areosa SA (2003). "Folic acid with or without vitamin B12 for cognition and dementia". Cochrane Database Syst Rev (4): CD004514. doi:. PMID 14584018.
- Sun Y, Lu CJ, Chien KL, Chen ST, Chen RC (2007). "Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's disease: a 26-week, randomised, double-blind, placebo-controlled study in Taiwanese patients". Clin Ther 29 (10): 2204–14. doi:. PMID 18042476.
- Boothby LA, Doering PL (2005). "Vitamin C and vitamin E for Alzheimer's disease". Ann Pharmacother 39 (12): 2073–80. doi:. PMID 16227450.
- Gray SL, Anderson ML, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, Larson E (2008). "Antioxidant vitamin supplement use and risk of dementia or Alzheimer's disease in older adults". J Am Geriatr Soc 56 (2): 291–295. doi:. PMID 18047492.
- ↑ Curcumin in diet:
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ (2007). "Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model". Journal of Neurochemistry 102 (4): 1095–1104. doi:. PMID 17472706.
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM (2001). "The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse". Journal of Neuroscience 21 (21): 8370–8377. PMID 11606625.
- ↑ Rosendorff C, Beeri MS, Silverman JM (2007). "Cardiovascular risk factors for Alzheimer's disease". Am J Geriatr Cardiol 16 (3): 143–9. PMID 17483665.
- ↑ Patterson C, Feightner JW, Garcia A, Hsiung GY, MacKnight C, Sadovnick AD (February 2008). "Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease". CMAJ 178 (5): 548–56. doi:. PMID 18299540.
- ↑ Reiss AB, Wirkowski E (2007). "Role of HMG-CoA reductase inhibitors in neurological disorders : progress to date". Drugs 67 (15): 2111–20. PMID 17927279.
- ↑ Kuller LH (August 2007). "Statins and dementia". Curr Atheroscler Rep 9 (2): 154–61. PMID 17877925.
- ↑ Szekely CA, Breitner JC, Fitzpatrick AL, et al (January 2008). "NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type". Neurology 70 (1): 17–24. doi:. PMID 18003940.
- ↑ Craig MC, Murphy DG (October 2007). "Estrogen: effects on normal brain function and neuropsychiatric disorders". Climacteric 10 Suppl 2: 97–104. doi:. PMID 17882683.
- ↑ Mori K, Takeda M (September 2007). "[Hormone replacement Up-to-date. Hormone replacement therapy and brain function]" (in Japanese). Clin Calcium 17 (9): 1349–54. doi:. PMID 17767023.
- ↑ Birks J, Grimley Evans J (2007). "Ginkgo biloba for cognitive impairment and dementia". Cochrane Database Syst Rev (2): CD003120. doi:. PMID 17443523. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD003120/frame.html. Retrieved on 2008-02-22.
- ↑ DeKosky ST, Williamson JD, Fitzpatrick AL et al. (2008). "Ginkgo biloba for Prevention of Dementia". Journal of the American Medical Association 300 (19): 2253-2262. http://jama.ama-assn.org/cgi/content/full/300/19/2253. Retrieved on 2008-11-18.
- ↑ Verghese J, Lipton RB, Katz MJ, et al (June 2003). "Leisure activities and the risk of dementia in the elderly". N. Engl. J. Med. 348 (25): 2508–16. doi:. PMID 12815136.
- ↑ Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS (2006). "The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study". Lancet Neurol 5 (5): 406–412. doi:. PMID 16632311.
- ↑ Bialystok E, Craik FIM, Freedman M (2007). "Bilingualism as a protection against the onset of symptoms of dementia". Neuropsychologia 42 (2): 459–464. doi:.
- ↑ Davanipour Z, Tseng CC, Lee PJ, Sobel E (2007). "A case-control study of occupational magnetic field exposure and Alzheimer's disease: results from the California Alzheimer's Disease Diagnosis and Treatment Centers". BMC Neurol 7: 13. doi:. PMID 17559686.
- ↑ Qiu C, Fratiglioni L, Karp A, Winblad B, Bellander T (November 2004). "Occupational exposure to electromagnetic fields and risk of Alzheimer's disease". Epidemiology 15 (6): 687–94. PMID 15475717. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=1044-3983&volume=15&issue=6&spage=687. Retrieved on 2008-09-22.
- ↑ Shcherbatykh I, Carpenter DO (May 2007). "The role of metals in the etiology of Alzheimer's disease". J. Alzheimers Dis. 11 (2): 191–205. PMID 17522444.
- ↑ Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF (July 2000). "Relation between aluminum concentrations in drinking water and Alzheimer's disease: an 8-year follow-up study". Am. J. Epidemiol. 152 (1): 59–66. PMID 10901330.
- ↑ Kukull WA, Larson EB, Bowen JD, et al (June 1995). "Solvent exposure as a risk factor for Alzheimer's disease: a case-control study". Am. J. Epidemiol. 141 (11): 1059–71; discussion 1072–9. PMID 7771442.
- ↑ Santibáñez M, Bolumar F, García AM (2007). "Occupational risk factors in Alzheimer's disease: a review assessing the quality of published epidemiological studies". Occupational and Environmental Medicine 64 (11): 723–732. doi:. PMID 17525096.
- ↑ Seidler A, Geller P, Nienhaus A, et al (February 2007). "Occupational exposure to low frequency magnetic fields and dementia: a case-control study". Occup Environ Med 64 (2): 108–14. doi:. PMID 17043077.
- ↑ Rondeau V (2002). "A review of epidemiologic studies on aluminum and silica in relation to Alzheimer's disease and associated disorders". Rev Environ Health 17 (2): 107–21. PMID 12222737.
- ↑ Martyn CN, Coggon DN, Inskip H, Lacey RF, Young WF (May 1997). "Aluminum concentrations in drinking water and risk of Alzheimer's disease". Epidemiology 8 (3): 281–6. PMID 9115023.
- ↑ Graves AB, Rosner D, Echeverria D, Mortimer JA, Larson EB (September 1998). "Occupational exposures to solvents and aluminium and estimated risk of Alzheimer's disease". Occup Environ Med 55 (9): 627–33. PMID 9861186.
- ↑ Geula C, Mesulam MM (1995). "Cholinesterases and the pathology of Alzheimer disease". Alzheimer Dis Assoc Disord 9 Suppl 2: 23–28. PMID 8534419.
- ↑ Stahl SM (2000). "The new cholinesterase inhibitors for Alzheimer's disease, Part 2: illustrating their mechanisms of action". J Clin Psychiatry 61 (11): 813–814. PMID 11105732.
- ↑ "Donepezil". US National Library of Medicine (Medline Plus) (2007-01-08). Retrieved on 2008-03-20.
- ↑ "Galantamine". US National Library of Medicine (Medline Plus) (2007-01-08). Retrieved on 2008-03-20.
- ↑ "Rivastigmine". US National Library of Medicine (Medline Plus) (2007-01-08). Retrieved on 2008-03-20.
- ↑ "Rivastigmine Transdermal". US National Library of Medicine (Medline Plus) (2007-01-08). Retrieved on 2008-03-20.
- ↑ Birks J (2006). "Cholinesterase inhibitors for Alzheimer's disease". Cochrane Database Syst Rev (1): CD005593. doi:. PMID 16437532.
- ↑ Birks J, Harvey RJ (2006). "Donepezil for dementia due to Alzheimer's disease". Cochrane Database Syst Rev (1): CD001190. doi:. PMID 16437430.
- ↑ Raschetti R, Albanese E, Vanacore N, Maggini M (2007). "Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials". PLoS Med 4 (11): e338. doi:. PMID 18044984.
- ↑ Acetylcholinesterase inhibitors prescribing information:
- "Aricept Prescribing information" (PDF). Eisai and Pfizer. Retrieved on 2008-08-18.
- "Razadyne ER U.S. Full Prescribing Information" (PDF). Ortho-McNeil Neurologics. Retrieved on 2008-02-19.
- "Exelon ER U.S. Prescribing Information" (PDF). Novartis Pharmaceuticals. Retrieved on 2008-02-19.
- "Exelon U.S. Prescribing Information" (PDF). Novartis Pharmaceuticals. Retrieved on 2008-02-21.
- "Exelon Warning Letter" (PDF). FDA.
- ↑ 138.0 138.1 Lipton SA (2006). "Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond". Nat Rev Drug Discov 5 (2): 160–170. doi:. PMID 16424917.
- ↑ "Memantine". US National Library of Medicine (Medline) (2004-01-04). Retrieved on 2008-03-22.
- ↑ Areosa Sastre A, McShane R, Sherriff F (2004). "Memantine for dementia". Cochrane Database Syst Rev (4): CD003154. doi:. PMID 15495043.
- ↑ "Namenda Prescribing Information" (PDF). Forest Pharmaceuticals. Retrieved on 2008-02-19.
- ↑ Raina P, Santaguida P, Ismaila A, et al (2008). "Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline". Annals of Internal Medicine 148 (5): 379–397. PMID 18316756.
- ↑ Antipsychotics use:
- Ballard C, Waite J (2006). "The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease". Cochrane Database Syst Rev (1): CD003476. doi:. PMID 16437455.
- Ballard C, Lana MM, Theodoulou M, et al (2008). "A Randomised, Blinded, Placebo-Controlled Trial in Dementia Patients Continuing or Stopping Neuroleptics (The DART-AD Trial)". PLoS Med. 5 (4): e76. doi:. PMID 18384230.
- Sink KM, Holden KF, Yaffe K (2005). "Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence". JAMA 293 (5): 596–608. doi:. PMID 15687315.
- ↑ 144.0 144.1 144.2 144.3 144.4 144.5 144.6 "Practice Guideline for the Treatment of Patients with Alzheimer's disease and Other Dementias" (PDF). American Psychiatric Association (October 2007). doi:10.1176/appi.books.9780890423967.152139. Retrieved on 2007-12-28.
- ↑ Bottino CM, Carvalho IA, Alvarez AM, et al (2005). "Cognitive rehabilitation combined with drug treatment in Alzheimer's disease patients: a pilot study". Clin Rehabil 19 (8): 861–869. doi:. PMID 16323385.
- ↑ Doody RS, Stevens JC, Beck C, et al (2001). "Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology 56 (9): 1154–1166. PMID 11342679.
- ↑ Hermans DG, Htay UH, McShane R (2007). "Non-pharmacological interventions for wandering of people with dementia in the domestic setting". Cochrane Database Syst Rev (1): CD005994. doi:. PMID 17253573.
- ↑ Robinson L, Hutchings D, Dickinson HO, et al (2007). "Effectiveness and acceptability of non-pharmacological interventions to reduce wandering in dementia: a systematic review". Int J Geriatr Psychiatry 22 (1): 9–22. doi:. PMID 17096455.
- ↑ Woods B, Spector A, Jones C, Orrell M, Davies S (2005). "Reminiscence therapy for dementia". Cochrane Database Syst Rev (2): CD001120. doi:. PMID 15846613.
- ↑ Peak JS, Cheston RI (2002). "Using simulated presence therapy with people with dementia". Aging Ment Health 6 (1): 77–81. doi:. PMID 11827626.
- ↑ Camberg L, Woods P, Ooi WL, et al (1999). "Evaluation of Simulated Presence: a personalised approach to enhance well-being in persons with Alzheimer's disease". J Am Geriatr Soc 47 (4): 446–452. PMID 10203120.
- ↑ Neal M, Briggs M (2003). "Validation therapy for dementia". Cochrane Database Syst Rev (3): CD001394. doi:. PMID 12917907.
- ↑ Chung JC, Lai CK, Chung PM, French HP (2002). "Snoezelen for dementia". Cochrane Database Syst Rev (4): CD003152. doi:. PMID 12519587.
- ↑ Spector A, Orrell M, Davies S, Woods B (2000). "Withdrawn: Reality orientation for dementia". Cochrane Database Syst Rev (3): CD001119. doi:. PMID 17636652.
- ↑ Spector A, Thorgrimsen L, Woods B, et al (2003). "Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial". Br J Psychiatry 183: 248–254. doi:. PMID 12948999.
- ↑ Gitlin LN, Corcoran M, Winter L, Boyce A, Hauck WW (February 2001). "A randomized, controlled trial of a home environmental intervention: effect on efficacy and upset in caregivers and on daily function of persons with dementia". Gerontologist 41 (1): 4–14. PMID 11220813. http://gerontologist.gerontologyjournals.org/cgi/pmidlookup?view=long&pmid=11220813. Retrieved on 2008-07-15.
- ↑ Gitlin LN, Hauck WW, Dennis MP, Winter L (March 2005). "Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer's disease and related disorders". J. Gerontol. A Biol. Sci. Med. Sci. 60 (3): 368–74. PMID 15860476.
- ↑ "Treating behavioral and psychiatric symptoms". Alzheimer's Association (2006). Retrieved on 2006-09-25.
- ↑ Dunne TE, Neargarder SA, Cipolloni PB, Cronin-Golomb A (2004). "Visual contrast enhances food and liquid intake in advanced Alzheimer's disease". Clinical Nutrition 23 (4): 533–538. doi:. PMID 15297089.
- ↑ Dudek, Susan G. (2007). Nutrition essentials for nursing practice. Hagerstown, Maryland: Lippincott Williams & Wilkins. pp. 360. ISBN 0-7817-6651-6. http://books.google.com/books?id=01zo6yf0IUEC&pg=PA360&dq=alzheimer%27s+chew&lr=&client=firefox-a&sig=ACfU3U05ChdcpPonim4W-Z1HIJdPQ93l7Q. Retrieved on 2008-08-19.
- ↑ Dennehy C (2006). "Analysis of patients' rights: dementia and PEG insertion". Br J Nurs 15 (1): 18–20. PMID 16415742.
- ↑ Chernoff R (April 2006). "Tube feeding patients with dementia". Nutr Clin Pract 21 (2): 142–6. PMID 16556924.
- ↑ Medical issues:
- Head B (January 2003). "Palliative care for persons with dementia". Home Healthc Nurse 21 (1): 53–60; quiz 61. PMID 12544465.
- Friedlander AH, Norman DC, Mahler ME, Norman KM, Yagiela JA (September 2006). "Alzheimer's disease: psychopathology, medical management and dental implications". J Am Dent Assoc 137 (9): 1240–51. PMID 16946428.
- Belmin J (2007). "Practical guidelines for the diagnosis and management of weight loss in Alzheimer's disease: a consensus from appropriateness ratings of a large expert panel". J Nutr Health Aging 11 (1): 33–7. PMID 17315078.
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello M, Teri L (October 2003). "Training caregivers to change the sleep hygiene practices of patients with dementia: the NITE-AD project". J Am Geriatr Soc 51 (10): 1455–60. PMID 14511168.
- Perls TT, Herget M (December 1995). "Higher respiratory infection rates on an Alzheimer's special care unit and successful intervention". J Am Geriatr Soc 43 (12): 1341–4. PMID 7490383.
- ↑ Shega JW, Levin A, Hougham GW, et al (April 2003). "Palliative Excellence in Alzheimer Care Efforts (PEACE): a program description". J Palliat Med 6 (2): 315–20. doi:. PMID 12854952.
- ↑ 165.0 165.1 Bowen JD, Malter AD, Sheppard L, et al (August 1996). "Predictors of mortality in patients diagnosed with probable Alzheimer's disease". Neurology 47 (2): 433–9. PMID 8757016.
- ↑ 166.0 166.1 Dodge HH, Shen C, Pandav R, DeKosky ST, Ganguli M (February 2003). "Functional transitions and active life expectancy associated with Alzheimer disease". Arch. Neurol. 60 (2): 253–9. PMID 12580712.
- ↑ Larson EB, Shadlen MF, Wang L, et al (April 2004). "Survival after initial diagnosis of Alzheimer disease". Ann. Intern. Med. 140 (7): 501–9. PMID 15068977.
- ↑ Jagger C, Clarke M, Stone A (January 1995). "Predictors of survival with Alzheimer's disease: a community-based study". Psychol Med 25 (1): 171–7. PMID 7792352.
- ↑ 169.0 169.1 Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST (May 2005). "Alzheimer disease and mortality: a 15-year epidemiological study". Arch. Neurol. 62 (5): 779–84. doi:. PMID 15883266.
- ↑ 170.0 170.1 170.2 Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Román GC (January 2008). "Incidence and subtypes of dementia in three elderly populations of central Spain". J. Neurol. Sci. 264 (1–2): 63–72. doi:. PMID 17727890.
- ↑ 171.0 171.1 171.2 Di Carlo A, Baldereschi M, Amaducci L, et al (January 2002). "Incidence of dementia, Alzheimer's disease, and vascular dementia in Italy. The ILSA Study". J Am Geriatr Soc 50 (1): 41–8. PMID 12028245.
- ↑ Andersen K, Launer LJ, Dewey ME, et al (December 1999). "Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group". Neurology 53 (9): 1992–7. PMID 10599770.
- ↑ 2000 U.S. estimates:
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA (August 2003). "Alzheimer disease in the US population: prevalence estimates using the 2000 census". Arch. Neurol. 60 (8): 1119–22. doi:. PMID 12925369.
- "Profiles of general demographic characteristics, 2000 census of population and housing, United States" (PDF). U.S. Census Bureau (2001). Retrieved on 2008-08-27.
- ↑ 174.0 174.1 Ferri CP, Prince M, Brayne C, et al (December 2005). "Global prevalence of dementia: a Delphi consensus study" (PDF). Lancet 366 (9503): 2112–7. doi:. PMID 16360788. http://www.sbgg.org.br/profissional/artigos/pdf/demencia_mundo.pdf. Retrieved on 2008-06-13.
- ↑ World Health Organization (2006). Neurological Disorders: Public Health Challenges. Switzerland: World Health Organization. pp. 204–207. ISBN 978-92-4-156336-9. http://www.who.int/mental_health/neurology/neurodiso/en/index.html.
- ↑ 176.0 176.1 Berchtold NC, Cotman CW (1998). "Evolution in the conceptualization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s". Neurobiol. Aging 19 (3): 173–89. PMID 9661992.
- ↑ Auguste D.:
- Alzheimer Alois (1907). "Über eine eigenartige Erkrankung der Hirnrinde [About a peculiar disease of the cerebral cortex]" (in (German)). Allgemeine Zeitschrift fur Psychiatrie und Psychisch-Gerichtlich Medizin 64 (1–2): 146–148.
- Alzheimer Alois (1987). "About a peculiar disease of the cerebral cortex. By Alois Alzheimer, 1907 (Translated by L. Jarvik and H. Greenson)". Alzheimer Dis Assoc Disord 1 (1): 3–8. PMID 3331112.
- Maurer Ulrike, Maurer Konrad (2003). Alzheimer: the life of a physician and the career of a disease. New York: Columbia University Press. pp. 270. ISBN 0-231-11896-1.
- ↑ Kraepelin Emil, Diefendorf A. Ross (translated by) (2007-01-17). Clinical Psychiatry: A Textbook For Students And Physicians (Reprint). Kessinger Publishing. pp. 568. ISBN 1-4325-0833-4.
- ↑ Katzman Robert, Terry Robert D, Bick Katherine L (editors) (1978). Alzheimer's disease: senile dementia and related disorders. New York: Raven Press. pp. 595. ISBN 0-89004-225-X.
- ↑ Boller F, Forbes MM (June 1998). "History of dementia and dementia in history: an overview". J. Neurol. Sci. 158 (2): 125–33. PMID 9702682.
- ↑ Amaducci LA, Rocca WA, Schoenberg BS (November 1986). "Origin of the distinction between Alzheimer's disease and senile dementia: how history can clarify nosology". Neurology 36 (11): 1497–9. PMID 3531918.
- ↑ Allegri RF, Butman J, Arizaga RL, et al (August 2007). "Economic impact of dementia in developing countries: an evaluation of costs of Alzheimer-type dementia in Argentina". Int Psychogeriatr 19 (4): 705–18. doi:. PMID 16870037.
- ↑ Suh GH, Knapp M, Kang CJ (August 2006). "The economic costs of dementia in Korea, 2002". Int J Geriatr Psychiatry 21 (8): 722–8. doi:. PMID 16858741.
- ↑ Wimo A, Jonsson L, Winblad B (2006). "An estimate of the worldwide prevalence and direct costs of dementia in 2003". Dement Geriatr Cogn Disord 21 (3): 175–81. doi:. PMID 16401889.
- ↑ 185.0 185.1 185.2 Moore MJ, Zhu CW, Clipp EC (July 2001). "Informal costs of dementia care: estimates from the National Longitudinal Caregiver Study". J Gerontol B Psychol Sci Soc Sci 56 (4): S219–28. PMID 11445614.
- ↑ Jönsson L, Eriksdotter Jönhagen M, Kilander L, et al (May 2006). "Determinants of costs of care for patients with Alzheimer's disease". Int J Geriatr Psychiatry 21 (5): 449–59. doi:. PMID 16676288.
- ↑ "The MetLife study of Alzheimer’s disease: The caregiving experience" (PDF). MetLife Mature Market Institute (August 2006). Retrieved on 2008-02-12.
- ↑ 188.0 188.1 Zhu CW, Sano M (2006). "Economic considerations in the management of Alzheimer's disease". Clin Interv Aging 1 (2): 143–54. PMID 18044111.
- ↑ Gaugler JE, Kane RL, Kane RA, Newcomer R (April 2005). "Early community-based service utilization and its effects on institutionalization in dementia caregiving". Gerontologist 45 (2): 177–85. PMID 15799982.
- ↑ Ritchie K, Lovestone S (November 2002). "The dementias". Lancet 360 (9347): 1759–66. doi:. PMID 12480441.
- ↑ Brodaty H, Hadzi-Pavlovic D (September 1990). "Psychosocial effects on carers of living with persons with dementia". Aust N Z J Psychiatry 24 (3): 351–61. PMID 2241719.
- ↑ Donaldson C, Tarrier N, Burns A (April 1998). "Determinants of carer stress in Alzheimer's disease". Int J Geriatr Psychiatry 13 (4): 248–56. PMID 9646153.
- ↑ "The MetLife Study of Alzheimer’s Disease: The Caregiving Experience" (PDF). MetLife Mature Market Institute (August 2006). Retrieved on 2008-02-12.
- ↑ Pusey H, Richards D (May 2001). "A systematic review of the effectiveness of psychosocial interventions for carers of people with dementia". Aging Ment Health 5 (2): 107–19. PMID 11511058.
- ↑ Garrard P, Maloney LM, Hodges JR, Patterson K (February 2005). "The effects of very early Alzheimer's disease on the characteristics of writing by a renowned author". Brain 128 (Pt 2): 250–60. doi:. PMID 15574466.
- ↑ Sherman FT (September 2004). "Did President Reagan have mild cognitive impairment while in office? Living longer with Alzheimer's Disease". Geriatrics 59 (9): 11, 15. PMID 15461232.
- ↑ "Hungary legend Puskas dies at 79". BBC News (2006-11-17). Retrieved on 2008-01-25.
- ↑ "Prime Ministers in History: Harold Wilson". London: 10 Downing Street. Retrieved on 2008-08-18.
- ↑ ""Mi padre no reconoció al Rey pero notó el cariño"". Madrid: El País (2008). Retrieved on 2008-10-01.
- ↑ "Chicago Rita Hayworth Gala". Alzheimer’s Association (2007). Retrieved on 2008-01-25.
- ↑ "Charlton Heston has Alzheimer's symptoms". CNN (2002-08-09). Retrieved on 2008-01-25.
- ↑ Pauli Michelle (2007-12-12). "Pratchett announces he has Alzheimer's", Guardian News and Media. Retrieved on 2008-08-18.
- ↑ "Iris". IMDB (2002-01-18). Retrieved on 2008-01-24.
- ↑ Bayley John (2000). Iris: a memoir of Iris Murdoch. London: Abacus. ISBN 9780349112152. OCLC 41960006.
- ↑ "The notebook". IMDB. Retrieved on 2008-02-22.
- ↑ Sparks Nicholas (1996). The notebook. Thorndike, Maine: Thorndike Press. pp. 268. ISBN 078620821X.
- ↑ "Thanmathra". Webindia123.com. Retrieved on 2008-01-24.
- ↑ "Ashita no kioku". IMDB. Retrieved on 2008-01-24.
- ↑ Ogiwara Hiroshi (2004) (in (Japanese)). Ashita no Kioku. Tōkyō: Kōbunsha. ISBN 9784334924461. OCLC 57352130.
- ↑ Munro Alice (2001). Hateship, Friendship, Courtship, Loveship, Marriage: Stories. New York: A.A. Knopf. ISBN 9780375413001. OCLC 46929223.
- ↑ Malcolm and Barbara:
- "Malcolm and Barbara: A love story". Dfgdocs. Retrieved on 2008-01-24.
- "Malcolm and Barbara: A love story". BBC Cambridgeshire. Retrieved on 2008-03-02.
- "Alzheimer's film-maker to face ITV lawyers", Guardian Media (2007-08-07). Retrieved on 2008-01-24.
- ↑ "Clinical Trials. Found 459 studies with search of: alzheimer". US National Institutes of Health. Retrieved on 2008-03-23.
- ↑ Vaccination:
- Hawkes CA, McLaurin J (November 2007). "Immunotherapy as treatment for Alzheimer's disease". Expert Rev Neurother 7 (11): 1535–48. doi:. PMID 17997702.
- Solomon B (June 2007). "Clinical immunologic approaches for the treatment of Alzheimer's disease". Expert Opin Investig Drugs 16 (6): 819–28. doi:. PMID 17501694.
- Woodhouse A, Dickson TC, Vickers JC (2007). "Vaccination strategies for Alzheimer's disease: A new hope?". Drugs Aging 24 (2): 107–19. PMID 17313199.
- ↑ "Study Evaluating ACC-001 in Mild to Moderate Alzheimers Disease Subjects". Clinical Trial. US National Institutes of Health (2008-03-11). Retrieved on 2008-06-05.
- ↑ "Study Evaluating Safety, Tolerability, and Immunogenicity of ACC-001 in Subjects With Alzheimer's Disease". US National Institutes of Health. Retrieved on 2008-06-05.
- ↑ "Alzheimer's Disease Vaccine Trial Suspended on Safety Concern". Medpage Today (2008-04-18). Retrieved on 2008-06-14.
- ↑ "Bapineuzumab in Patients With Mild to Moderate Alzheimer's Disease/ Apo_e4 non-carriers". Clinical Trial. US National Institutes of Health (2008-02-29). Retrieved on 2008-03-23.
- ↑ "Safety, Tolerability and Efficacy Study to Evaluate Subjects With Mild Cognitive Impairment". Clinical Trial. US National Institutes of Health (2008-03-11). Retrieved on 2008-03-23.
- ↑ "Study Evaluating the Safety, Tolerability and Efficacy of PBT2 in Patients With Early Alzheimer's Disease". Clinical Trial. US National Institutes of Health (2008-01-13). Retrieved on 2008-03-23.
- ↑ Etanercept research:
- Tobinick E, Gross H, Weinberger A, Cohen H (2006). "TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study". MedGenMed 8 (2): 25. PMID 16926764.
- Griffin WS (2008). "Perispinal etanercept: potential as an Alzheimer therapeutic". J Neuroinflammation 5: 3. doi:. PMID 18186919.
- Tobinick E (December 2007). "Perispinal etanercept for treatment of Alzheimer's disease". Curr Alzheimer Res 4 (5): 550–2. PMID 18220520.
- ↑ Wischik Claude M, Bentham Peter, Wischik Damon J, Seng Kwang Meng (July 2008). "Tau aggregation inhibitor (TAI) therapy with remberTM arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks". Alzheimer's & Dementia (Alzheimer’s Association) 4 (4): T167. doi:. http://www.abstractsonline.com/viewer/viewAbstractPrintFriendly.asp?CKey={E7C717CF-8D73-41E0-8DB0-FA92205978CD}&SKey={68E04DB5-AB1C-4F7B-9511-DA3173F4F755}&MKey={CFC5F7C6-CB6A-40C4-BC87-B30C9E64B1CC}&AKey={50E1744A-0C52-45B2-BF85-2A798BF24E02}. Retrieved on 2008-07-30.
- ↑ Harrington Charles, Rickard Janet E, Horsley David, et al (July 2008). "Methylthioninium chloride (MTC) acts as a Tau aggregation inhibitor (TAI) in a cellular model and reverses Tau pathology in transgenic mouse models of Alzheimer's disease". Alzheimer's & Dementia (Alzheimer’s Association) 4: T120–T121. doi:.
- ↑ Doody RS, Gavrilova SI, Sano M, et al (July 2008). "Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study". Lancet 372 (9634): 207–15. doi:. PMID 18640457.
Further reading
- Cummings JL, Frank JC, Cherry D, Kohatsu ND, Kemp B, Hewett L, Mittman B (2002). "Guidelines for managing Alzheimer's disease: Part I. Assessment". American Family Physician 65 (11): 2263–2272. PMID 12074525. http://www.aafp.org/afp/20020601/2263.html.
- Cummings JL, Frank JC, Cherry D, Kohatsu ND, Kemp B, Hewett L, Mittman B (2002). "Guidelines for managing Alzheimer's disease: Part II. Treatment". American Family Physician 65 (12): 2525–2534. PMID 12086242. http://www.aafp.org/afp/20020615/2525.html.
- "Genes, lifestyles, and crossword puzzles: Can Alzheimer's disease be prevented?" (PDF). US Department of Health and Human Services, National Institute on Aging. Retrieved on 2008-02-29.
- Russell D, Barston S, White M (2007-12-19). "Alzheimer’s Behavior Management: Learn to manage common behavior problems". helpguide.org. Retrieved on 2008-02-29.
- Rodgers AB (2003). Alzheimer's Disease: Unraveling the Mystery. National Institute on Aging. http://www.nia.nih.gov/Alzheimers/Publications/UnravelingTheMystery. Retrieved on 2008-08-22.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||