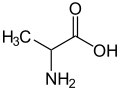
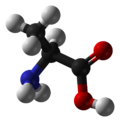
Alanine
| Alanine | |
|---|---|
 |
 |
| IUPAC name | (S)-2-aminopropanoic acid |
| Identifiers | |
| CAS number | 56-41-7 |
| PubChem | |
| SMILES |
|
| Properties | |
| Molecular formula | C3H7NO2 |
| Molar mass | 89.09 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Alanine (abbreviated as Ala or A)[1] is an α-amino acid with the chemical formula CH3CH(NH2)COOH. The L-isomer is one of the 20 proteinogenic amino acids, i.e. the building blocks of proteins. Its codons are GCU, GCC, GCA, and GCG. It is classified as a non-polar amino acid. L-alanine is second only to leucine, accounting for 7.8% of the primary structure in a sample of 1,150 proteins.[2] D-alanine occurs in bacterial cell walls and in some peptide antibiotics.
Contents |
Structure
The α-carbon atom of alanine is bound with a methyl group (-CH3), making it one of the simplest α-amino acids with respect to molecular structure and also resulting in alanine being classified as an aliphatic amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function.
Sources
Dietary Sources
Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained directly through the diet. Alanine is found in a wide variety of foods, but is particularly concentrated in meats.
Good sources of alanine include:
- Animal sources: meat, seafood, caseinate, dairy products, eggs, fish, gelatin, lactalbumin
- Vegetarian sources: beans, nuts, seeds, soy, whey, brewer's yeast, brown rice bran, corn, legumes, whole grains.
Biosynthesis
Alanine can be manufactured in the body from pyruvate and branched chain amino acids such as valine, leucine, and isoleucine.
Alanine is most commonly produced by reductive amination of pyruvate. Because transamination reactions are readily reversible and pyruvate pervasive, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle. It also arises together with lactate and generates glucose from protein via the alanine cycle.
Chemical Synthesis
Racemic alanine can be prepared via the condensation of acetaldehyde with ammonium chloride in the presence of potassium cyanide by the Strecker reaction.[3]
Physiological function
As a carrier of ammonia and of the carbon skeleton of pyruvate in alanine cycle
Alanine plays a key role in glucose-alanine cycle between tissues and liver. In muscle and other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate by transamination. Glutamate can then transfer its amino group through the action of alanine aminotransferase to pyruvate, a product of muscle glycolysis, forming alanine and alpha-ketoglutarate. The alanine formed is passed into the blood and transported to the liver. A reverse of the alanine aminotransferase reaction takes place in liver. Pyruvate regenerated forms glucose through gluconeogenesis, which returns to muscle through the circulation system. Glutamate in the liver enters mitochondria and degrades into ammonium ion through the action of glutamate dehydrogenase, which in turn participate in the urea cycle to form urea.[4]
Glucose-alanine cycle enables pyruvate and glutamate to be removed from muscle and find their ways to liver. Glucose is able to be regenerated from pyruvate and returned to muscle. The energetic burden of gluconeogenesis is thus imposed on the liver instead of the muscle. All available ATP in muscle is devoted to muscle contraction.[4]
Link to hypertension
An international study led by Imperial College London found a correlation between high levels of alanine and higher blood pressure, energy intake, cholesterol levels, and body mass index.[5]
Chemical properties
Free radical stability
The deamination of an alanine molecule produces a stable alkyl free radical, CH3C•HCOO–. Deamination can be induced in solid or aqueous alanine by radiation.[6]
This property of alanine is used in dosimetric measurements in radiotherapy. When normal alanine is irradiated, the radiation causes certain alanine molecules to become free radicals, and, as these radicals are stable, the free radical content can later be measured in order to find out how much radiation the alanine was exposed to. In this way, one can be assured that complex radiotherapy treatment plans will deliver the intended pattern of radiation dose.
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved on 2007-05-17.
- ↑ Doolittle, R. F.; & Fasman G.D. (ed.) (1989). "Redundancies in protein sequences" in Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press, pp. 599-623.
- ↑ Kendall, E. C.; McKenzie, B. F. “dl-Alanine” Organic Syntheses, Collected Volume 1, p.21 (1941). http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf
- ↑ 4.0 4.1 Nelson, D. L. & Cox, M. M. (2005). Lehninger Principles of Biochemistry, 4th Edition. New York: W. H. Freeman and Company, pp. 684-685. ISBN 0-7167-4339-6.
- ↑ 'Metabolic fingerprint' linked to high blood pressure - Telegraph
- ↑ Journal of Radioanalytical and Nuclear Chemistry, 1998, 232(1-2), pp. 139-141. DOI 10.1007/BF02383729 http://www.springerlink.com/content/j050542210k251ru/
See also
- carbamic acid
- glycine
|
||||||||||||||