Earth's atmosphere
 Atmospheric gases scatter blue light more than other wavelengths, giving the Earth a blue halo when seen from space |
|
| Components of dry air | (volume) |
| Nitrogen | 78.0842% |
| Oxygen | 20.9463% |
| Argon | 0.9342% |
| Carbon dioxide | 0.0384% |
| Other | 0.0020% |

The Earth's atmosphere is a layer of gases surrounding the planet Earth that is retained by the Earth's gravity. Dry air contains roughly (by molar content – equivalent to volume, for gases) 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.038% carbon dioxide, and trace amounts of other gases; but air also contains a variable amount of water vapor, on average around 1%. This mixture of gases is commonly known as air. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention (greenhouse effect), and reducing temperature extremes between day and night.
There is no definite boundary between the atmosphere and outer space. It slowly becomes thinner and fades into space. Three quarters of the atmosphere's mass is within 11 km of the planetary surface. An altitude of 120 km (~75 miles or 400,000 ft) marks the boundary where atmospheric effects become noticeable during re-entry. The Kármán line, at 100 km (62 miles or 328,000 ft), is also frequently regarded as the boundary between atmosphere and outer space.
Contents |
Temperature and layers
The temperature of the Earth's atmosphere varies with altitude; the mathematical relationship between temperature and altitude varies among five different atmospheric layers (ordered highest to lowest, the ionosphere is part of the thermosphere):
- Exosphere: from 500 – 1000 km (300 – 600 mi) up to 10,000 km (6,000 mi), free-moving particles that may migrate into and out of the magnetosphere or the solar wind.
- Ionosphere: the part of the atmosphere that is ionized by solar radiation. It plays an important part in atmospheric electricity and forms the inner edge of the magnetosphere. It has practical importance because, among other functions, it influences radio propagation to distant places on the Earth. It is located in the thermosphere and is responsible for auroras.
- Thermosphere: from 80 – 85 km (265,000 – 285,000 ft) to 640+ km (400+ mi), temperature increasing with height.
- Mesosphere: From the Greek word "μέσος" meaning middle. The mesosphere extends from about 50 km (160,000 ft) to the range of 80 to 85 km (265,000 – 285,000 ft), temperature decreasing with height. This is also where most meteors burn up when entering the atmosphere.
- Stratosphere: From the Latin word "stratus" meaning a spreading out. The stratosphere extends from the troposphere's 7 to 17 km (23,000 – 60,000 ft) range to about 50 km (160,000 ft). Temperature increases with height. The stratosphere contains the ozone layer, the part of the Earth's atmosphere which contains relatively high concentrations of ozone. "Relatively high" means a few parts per million—much higher than the concentrations in the lower atmosphere but still small compared to the main components of the atmosphere. It is mainly located in the lower portion of the stratosphere from approximately 15 to 35 km (50,000 – 115,000 ft) above Earth's surface, though the thickness varies seasonally and geographically.
- Troposphere: From the Greek word "τρέπω" meaning to turn or change. The troposphere is the lowest layer of the atmosphere; it begins at the surface and extends to between 7 km (23,000 ft) at the poles and 17 km (60,000 ft) at the equator, with some variation due to weather factors. The troposphere has a great deal of vertical mixing because of solar heating at the surface. This heating warms air masses, which makes them less dense so they rise. When an air mass rises, the pressure upon it decreases so it expands, doing work against the opposing pressure of the surrounding air. To do work is to expend energy, so the temperature of the air mass decreases. As the temperature decreases, water vapor in the air mass may condense or solidify, releasing latent heat that further uplifts the air mass. This process determines the maximum rate of decline of temperature with height, called the adiabatic lapse rate. The troposphere contains roughly 80% of the total mass of the atmosphere. Fifty percent of the total mass of the atmosphere is located in the lower 5.6 km of the troposphere.
The average temperature of the atmosphere at the surface of Earth is 15 °C (59 °F).[1][2]
Pressure and Thickness
- Barometric Formula: (used for airplane flight) barometric formula
- One mathematical model: NRLMSISE-00
The average atmospheric pressure, at sea level, is about 101.3 kilopascals (about 14.7 psi); total atmospheric mass is 5.1480×1018 kg [3].
Atmospheric pressure is a direct result of the total weight of the air above the point at which the pressure is measured. This means that air pressure varies with location and time, because the amount (and weight) of air above the earth varies with location and time. However the average mass of the air above a square meter of the earth's surface is known to the same high accuracy as the total air mass of 5148.0 teratonnes and area of the earth of 51007.2 megahectares, namely 5148.0/510.072 = 10.093 metric tonnes per square meter or 14.356 lbs (mass) per square inch. This is about 2.5% below the officially standardized unit atmosphere (1 atm) of 101.325 kPa or 14.696 psi, and corresponds to the mean pressure not at sea level but at the mean base of the atmosphere as contoured by the earth's terrain.
Were atmospheric density to remain constant with height the atmosphere would terminate abruptly at 7.81 km (25,600 ft). Instead it decreases with height, dropping by 50% at an altitude of about 5.6 km (18,000 ft). For comparison: the highest mountain, Mount Everest, is higher, at 8.8 km, which is why it is so difficult to climb without supplemental oxygen. This pressure drop is approximately exponential, so that pressure decreases by approximately half every 5.6 km (whence about 50% of the total atmospheric mass is within the lowest 5.6 km) and by 63.2 % 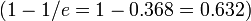 every 7.64 km, the average scale height of Earth's atmosphere below 70 km. However, because of changes in temperature, average molecular weight, and gravity throughout the atmospheric column, the dependence of atmospheric pressure on altitude is modeled by separate equations for each of the layers listed above.
every 7.64 km, the average scale height of Earth's atmosphere below 70 km. However, because of changes in temperature, average molecular weight, and gravity throughout the atmospheric column, the dependence of atmospheric pressure on altitude is modeled by separate equations for each of the layers listed above.
Even in the exosphere, the atmosphere is still present (as can be seen for example by the effects of atmospheric drag on satellites).
The equations of pressure by altitude in the above references can be used directly to estimate atmospheric thickness. However, the following published data are given for reference: [4]
- 50% of the atmosphere by mass is below an altitude of 5.6 km.
- 90% of the atmosphere by mass is below an altitude of 16 km. The common altitude of commercial airliners is about 10 km.
- 99.99997% of the atmosphere by mass is below 100 km. The highest X-15 plane flight in 1963 reached an altitude of 354,300 ft (108.0 km).
Therefore, most of the atmosphere (99.9997%) is below 100 km, although in the rarefied region above this there are auroras and other atmospheric effects.
Composition
Filtered air includes at least trace amounts of ten (or more) of the chemical elements. Substantial amounts of argon, nitrogen, and oxygen are present as elementary gases, as well as hydrogen (and additional oxygen) in water vapor (H2O). Much smaller or trace amounts of elementary helium, hydrogen, iodine, krypton, neon, and xenon are also present, as well as carbon in carbon dioxide (CO2), methane (CH4), and carbon monoxide (CO). Many additional elements from natural sources may be present in tiny amounts in an unfiltered air sample, including contributions from dust, pollen and spores, sea spray, vulcanism, and meteoroids. Various industrial pollutants are also now present in the air, such as chlorine (elementary or in compounds), fluorine (in compounds), elementary mercury, and sulfur (in compounds such as sulfur dioxide [SO2]).


| ppmv: parts per million by volume | |
| Gas | Volume |
|---|---|
| Nitrogen (N2) | 780,840 ppmv (78.084%) |
| Oxygen (O2) | 209,460 ppmv (20.946%) |
| Argon (Ar) | 9,340 ppmv (0.9340%) |
| Carbon dioxide (CO2) | 383 ppmv (0.0383%) |
| Neon (Ne) | 18.18 ppmv (0.001818%) |
| Helium (He) | 5.24 ppmv (0.000524%) |
| Methane (CH4) | 1.745 ppmv (0.0001745%) |
| Krypton (Kr) | 1.14 ppmv (0.000114%) |
| Hydrogen (H2) | 0.55 ppmv (0.000055%) |
| Not included in above dry atmosphere: | |
| Water vapor (H2O) | ~0.40% over full atmosphere, typically 1% to 4% near surface |
| Gas | Volume |
|---|---|
| nitrous oxide | 0.3 ppmv (0.00003%) |
| xenon | 0.09 ppmv (9x10-6%) |
| ozone | 0.0 to 0.07 ppmv (0%-7x10-6%) |
| nitrogen dioxide | 0.02 ppmv (2x10-6%) |
| iodine | 0.01 ppmv (1x10-6%) |
| carbon monoxide | trace |
| ammonia | trace |
ppmv
The composition figures above are by volume-fraction (V%), which for ideal gases is equal to mole-fraction (that is, the fraction of total molecules). Although the atmosphere is not an ideal gas, nonetheless the atmosphere behaves enough like an ideal gas that the volume-fraction is the same as the mole-fraction for the precision given.
By contrast, mass-fraction abundances of gases will differ from the volume values. The mean molar mass of air is 28.97 g/mol, while the molar mass of helium is 4.00, and krypton is 83.80. Thus helium is 5.2 ppm by volume-fraction, but 0.72 ppm by mass-fraction ([4/29] × 5.2 = 0.72), and krypton is 1.1 ppm by volume-fraction, but 3.2 ppm by mass-fraction ([84/29] × 1.1 = 3.2).
Heterosphere
Below the turbopause at an altitude of about 100 km (not far from the mesopause), the Earth's atmosphere has a more-or-less uniform composition (apart from water vapor) as described above; this constitutes the homosphere.[6] However, above about 100 km, the Earth's atmosphere begins to have a composition which varies with altitude. This is essentially because, in the absence of mixing, the density of a gas falls off exponentially with increasing altitude but at a rate which depends on the molar mass. Thus higher mass constituents, such as oxygen and nitrogen, fall off more quickly than lighter constituents such as helium, molecular hydrogen, and atomic hydrogen. Thus there is a layer, called the heterosphere, in which the earth's atmosphere has varying composition. As the altitude increases, the atmosphere is dominated successively by helium, molecular hydrogen, and atomic hydrogen. The precise altitude of the heterosphere and the layers it contains varies significantly with temperature.
In pre-history, the Sun's radiation caused a loss of the hydrogen, helium and other hydrogen-containing gases from early Earth, and Earth was devoid of an atmosphere. The first atmosphere was formed by outgassing of gases trapped in the interior of the early Earth, which still goes on today in volcanoes.[7]
Density and mass


The density of air at sea level is about 1.2 kg/m3 (1.2 g/L). Natural variations of the barometric pressure occur at any one altitude as a consequence of weather. This variation is relatively small for inhabited altitudes but much more pronounced in the outer atmosphere and space because of variable solar radiation.
The atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the barometric formula. More sophisticated models are used by meteorologists and space agencies to predict weather and orbital decay of satellites.
The average mass of the atmosphere is about 5 quadrillion metric tons or 1/1,200,000 the mass of Earth. According to the National Center for Atmospheric Research, "The total mean mass of the atmosphere is 5.1480×1018 kg with an annual range due to water vapor of 1.2 or 1.5×1015 kg depending on whether surface pressure or water vapor data are used; somewhat smaller than the previous estimate. The mean mass of water vapor is estimated as 1.27×1016 kg and the dry air mass as 5.1352 ±0.0003×1018 kg."
Opacity

The atmosphere has "windows" of low opacity, allowing the transmission of electromagnetic radiation. The optical window runs from around 300 nanometers (ultraviolet-C) at the short end up into the range the eye can use, the visible spectrum at roughly 400–700 nm, and continues up through the visual infrared to around 1100 nm, which is thermal infrared. There are also infrared and radio windows that transmit some infrared and radio waves. The radio window runs from about one centimeter to about eleven-meter waves.
Evolution of Earth's Atmosphere
- See also: History of Earth and Gaia hypothesis
The history of the Earth's atmosphere prior to one billion years ago is poorly understood and an active area of scientific research. The following discussion presents a plausible scenario.
The modern atmosphere is sometimes referred to as Earth's "third atmosphere", in order to distinguish the current chemical composition from two notably different previous compositions. The original atmosphere was primarily helium and hydrogen. Heat from the still-molten crust, and the sun, plus a probably enhanced solar wind, dissipated this atmosphere.
About 4.4 billion years ago, the surface had cooled enough to form a crust, still heavily populated with volcanoes which released steam, carbon dioxide, and ammonia. This led to the early "second atmosphere", which was primarily carbon dioxide and water vapor, with some nitrogen but virtually no oxygen. This second atmosphere had approximately 100 times as much gas as the current atmosphere, but as it cooled much of the carbon dioxide was dissolved in the seas and precipitated out as carbonates. The later "second atmosphere" contained largely nitrogen and carbon dioxide. However, simulations run at the University of Waterloo and University of Colorado in 2005 suggest that it may have had up to 40% hydrogen.[8] It is generally believed that the greenhouse effect, caused by high levels of carbon dioxide and methane, kept the Earth from freezing.
One of the earliest types of bacteria was the cyanobacteria. Fossil evidence indicates that bacteria shaped like these existed approximately 3.3 billion years ago and were the first oxygen-producing evolving phototropic organisms. They were responsible for the initial conversion of the earth's atmosphere from an anoxic state to an oxic state (that is, from a state without oxygen to a state with oxygen) during the period 2.7 to 2.2 billion years ago. Being the first to carry out oxygenic photosynthesis, they were able to produce oxygen while sequestering carbon dioxide in organic molecules, playing a major role in oxygenating the atmosphere.
Photosynthesising plants later evolved and continued releasing oxygen and sequestering carbon dioxide. Over time, excess carbon became locked in fossil fuels, sedimentary rocks (notably limestone), and animal shells. As oxygen was released, it reacted with ammonia to release nitrogen; in addition, bacteria would also convert ammonia into nitrogen. But most of the nitrogen currently present in the atmosphere results from sunlight-powered photolysis of ammonia released steadily over the aeons from volcanoes.
As more plants appeared, the levels of oxygen increased significantly, while carbon dioxide levels dropped. At first the oxygen combined with various elements (such as iron), but eventually oxygen accumulated in the atmosphere, contributing to Cambrian explosion and further evolution. With the appearance of an ozone layer (ozone is an allotrope of oxygen) lifeforms were better protected from ultraviolet radiation. This oxygen-nitrogen atmosphere is the "third atmosphere". Between 200 and 250 million years ago, up to 35% of the atmosphere was oxygen (as found in bubbles of ancient atmosphere preserved in amber).
This modern atmosphere has a composition which is enforced by oceanic blue-green algae as well as geological processes. O2 does not remain naturally free in an atmosphere but tends to be consumed (by inorganic chemical reactions, and by animals, bacteria, and even land plants at night), and CO2 tends to be produced by respiration and decomposition and oxidation of organic matter. Oxygen would vanish within a few million years by chemical reactions, and CO2 dissolves easily in water and would be gone in millennia if not replaced. Both are maintained by biological productivity and geological forces seemingly working hand-in-hand to maintain reasonably steady levels over millions of years.
Currently, anthropogenic greenhouse gases are increasing in the atmosphere and this is a causative factor in global warming.[9]
Air pollution

Air pollution is the human introduction into the atmosphere of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or damages the environment.[10] Stratospheric ozone depletion is believed to be caused by air pollution (chiefly from chlorofluorocarbons)
Worldwide air pollution is responsible for large numbers of deaths and cases of respiratory disease. Enforced air quality standards, like the Clean Air Act in the United States, have reduced the presence of some pollutants. While major stationary sources are often identified with air pollution, the greatest source of emissions is actually mobile sources, principally the automobile. Gases such as carbon dioxide, methane, and fluorocarbons contribute to global warming, and these gases, or excess amounts of some emitted from fossil fuel burning, have recently been identified by the United States and many other countries as pollutants.
Kyoto Protocol

As of April 2008, 178 states have signed and ratified the Kyoto Protocol to the United Nations Framework Convention on Climate Change, aimed at combating global warming.
The Kyoto Protocol is a protocol to the international Framework Convention on Climate Change with the objective of reducing greenhouse gases in an effort to prevent anthropogenic climate change.
It was adopted for use on 11 December 1997 by the 3rd Conference of the Parties, which was meeting in Kyoto, and it entered into force on 16 February 2005. As of May 2008, 182 parties have ratified the protocol.[11] Of these, 36 developed C.G. countries (plus the EU as a party in its own right) are required to reduce greenhouse gas emissions to the levels specified for each of them in the treaty (representing over 61.6% of emissions from Annex I countries),[11][12] with three more countries intending to participate.[13] One hundred thirty-seven (137) developing countries have ratified the protocol, including Brazil, China and India, but have no obligation beyond monitoring and reporting emissions. The United States is the only developed and industrialized western country that has not ratified the treaty but it is one of the significant greenhouse gas emitters.
See also
- Aerial perspective
- Air glow
- Airshed
- Atmosphere (for information on atmospheres in general)
- Atmospheric chemistry
- Atmospheric dispersion modeling
- Atmospheric electricity
- Atmospheric models
- Atmospheric Radiation Measurement (ARM) (in the U.S.)
- Atmospheric stratification
- Aviation
- Biosphere
- Compressed air
- Global dimming
- Historical temperature record
- Hydrosphere
- Intergovernmental Panel on Climate Change
- Lithosphere
- US Standard Atmosphere
References
- ↑ "Earth's Radiation Balance and Oceanic Heat Fluxes".
- ↑ "Coupled Model Intercomparison Project Control Run".
- ↑ The Mass of the Atmosphere: A Constraint on Global Analyses
- ↑ Lutgens, Frederick K. and Edward J. Tarbuck (1995) The Atmosphere, Prentice Hall, 6th ed., pp14-17, ISBN 0-13-350612-6
- ↑ Source for figures: Carbon dioxide, NASA Earth Fact Sheet, (updated 2007.01). Methane, IPCC TAR table 6.1, (updated to 1998). The NASA total was 17 ppmv over 100%, and CO2 was increased here by 15 ppmv. To normalize, N2 should be reduced by about 25 ppmv and O2 by about 7 ppmv.
- ↑ homosphere—AMS Glossary
- ↑ Vercheval, J. "The thermosphere: a part of the heterosphere". (offline, see Internet Archive copy)
- ↑ "Early Earth atmosphere favorable to life: study", University of Waterloo (2005-04-07). Retrieved on 2007-07-30.
- ↑ "Summary for Policymakers" (PDF). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change (2007-02-05).
- ↑ Starting from [1] Pollution - Definition from the Merriam-Webster Online Dictionary
- ↑ 11.0 11.1 "Kyoto Protocol: Status of Ratification" (PDF). United Nations Framework Convention on Climate Change (2008-05-13). Retrieved on 2008-07-01.
- ↑ "Climate Action Network Europe: Ratification Calendar". Retrieved on 30 October, 2006.
- ↑ Cyprus and Malta will participate indirectly through membership of the EU ETS. Kazakhstan has commented that it intends to become an Annex I party after its ratification of the Protocol]
External links

- NASA atmosphere models
- NASA's Earth Fact Sheet
- American Geophysical Union: Atmospheric Sciences
- Stuff in the Air Find out what the atmosphere contains.
- Layers of the Atmosphere
- Answers to several questions of curious kids related to Air and Atmosphere
- The AMS Glossary of Meteorology
- Paul Crutzen Interview Free video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust.
- Slides describing the Earth's modern atmosphere
|
|||||||||||
|
||||||||||
|
||||||||||||||||||||