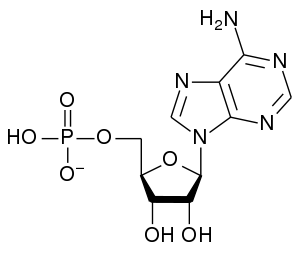
Adenosine monophosphate
| Adenosine monophosphate | |
|---|---|
 |
|
| IUPAC name | 5'-Adenylic acid |
| Identifiers | |
| CAS number | 61-19-8 |
| MeSH | |
| SMILES |
|
| Properties | |
| Molecular formula | C10H14N5O7P |
| Molar mass | 347.22 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide that is found in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine.
Contents |
Production and degradation
AMP can be produced during ATP synthesis by the enzyme adenylate kinase by combining two ADP molecules:
- 2 ADP → ATP + AMP
Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP:
- ADP → AMP + Pi
AMP can also be formed by hydrolysis of ATP into AMP and pyrophosphate:
- ATP → AMP + PPi
When RNA is broken down by living systems, nucleoside monophosphates, including adenosine monophosphate, are formed.
AMP can be regenerated to ATP as follows:
- AMP + ATP → 2 ADP (adenylate kinase in the opposite direction)
- ADP + Pi → ATP (this step is most often performed in aerobes by the ATP synthase during oxidative phosphorylation)
AMP can be converted into IMP by the enzyme myoadenylate deaminase, freeing an ammonia group.
In a catabolic pathway, adenosine monophosphate can be converted to uric acid, which is excreted from the body.
cAMP
AMP can also exist as a cyclic structure known as cyclic AMP (or cAMP). Within certain cells the enzyme adenylate cyclase makes cAMP from ATP, and typically this reaction is regulated by hormones such as adrenaline or glucagon. cAMP plays an important role in intracellular signaling.
Application as a bitterness suppressor
To human tastes, the bitterness-suppressing quality of AMP interprets as food seeming 'sweeter'. This makes lower-calorie food products more palatable, making AMP potentially a lucrative solution for food manufacturers as they respond to pressure from consumers and regulators concerned about social trends towards obesity.[1] AMP has been approved by the Food and Drug Administration as a 'Bitter Blocker' additive to foodstuffs.[2]
See also
- DNA
- Oligonucleotide
References
- ↑ Nucleotide compounds that block the bitter taste of oral compositions patent by Linguagen Corp)
- ↑ Bitter Blocker Backed
External links
|
|||||||||||||||||||||||||||