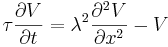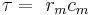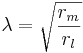Action potential
In neurophysiology, the action potential is a self-regenerating wave of electrochemical activity that allows nerve cells to carry a signal over a distance. It is the primary electrical signal generated by nerve cells, and arises from changes in the permeability of the nerve cell's axonal membranes to specific ions. Action potentials (also known as nerve impulses or spikes) are pulse-like waves of voltage that travel along several types of cell membranes.[1] The best-understood example of an action potential is that which is generated on the membrane of the axon of a neuron, but also appears in other types of excitable cells, such as cardiac muscle cells, and even plant cells.
A typical action potential is initiated at the axon hillock when the membrane potential is depolarized sufficiently (i.e. when its voltage is increased sufficiently). As the membrane potential is increased, both the sodium and potassium ion channels begin to open up. This increases both the inward sodium current and the balancing outward potassium current. For small voltage increases, the potassium current triumphs over the sodium current and the voltage returns to its normal resting value, typically −70 mV.[2] However, if the voltage increases past a critical threshold, typically 15 mV higher than the resting value, the sodium current dominates. This results in a runaway condition whereby the positive feedback from the sodium current activates even more sodium channels. Thus, the cell "fires", producing an action potential.[3]
Once initiated, the action potential travels through the axon.[4] Since the axon is insulated, the action potential can travel through it without significant signal decay. Nevertheless, to ensure the signal does not fail, regularly spaced patches, called the nodes of Ranvier, help to boost the signal. The process here is much the same as that at the axon hillock. The action potential depolarizes the membrane patch at the node of Ranvier, sparking another action potential there. In effect, the action potential is created afresh at each node of Ranvier. The axon then branches along its length, and the action potentials travel down each branch. At this point, the axon sheds its insulation, and instead, the action potential is propagated by the voltage activated sodium channels. Here, the inward current may not quite suffice to trigger a new action potential in some of these branches. The action potential may thus fail.[5] Action potentials that do reach the ends of the axon generally cause the release of a neurotransmitter into the synaptic cleft. This may combine with other inputs to provoke a new action potential in the post-synaptic neuron or muscle cell.
The principal ions involved in an action potential are sodium and potassium cations; sodium ions enter the cell, and potassium ions leave, restoring equilibrium. Relatively few ions need to cross the membrane for the membrane voltage to change drastically. The ions exchanged during an action potential, therefore, make a negligible change in the interior and exterior ionic concentrations. The few ions that do cross are pumped out again by the continual action of the sodium–potassium pump, which, with other ion transporters, maintains the normal ratio of ion concentrations across the membrane. Calcium cations and chloride anions are involved in a few types of action potentials, such as the cardiac action potential and the action potential in the single-celled alga Acetabularia, respectively.
Contents |
Biophysical and cellular context
Ions and the forces driving their motion

Electrical signals within biological organisms are generally driven by ions.[6] The most important cations for the action potential are sodium (Na+) and potassium. (K+),[7] Both of these are monovalent cations that carry a single positive charge. Action potentials can also involve calcium (Ca2+),[8] which is a divalent cation that carries a double positive charge. The chloride anion (Cl−) plays a major role in the action potentials of some algae,[9] but plays a negligible role in the action potentials of most animals.[10]
Ions cross the cell membrane under two influences: diffusion and electric fields. Lets start off with a simple example whereby two solutions are separated by a porous barrier. Let us further call these two solutions A and B. In this case, diffusion will ensure that they will eventually mix into equal solutions. This mixing occurs because of the difference in their concentrations. The region with high concentration will diffuse out towards the region with low concentration. To further the example, let solution A have 30 sodium ions and 30 chloride ions. Also, let solution B have only 20 sodium ions and 20 chloride ions. Assuming the barrier allows both types of ions to travel through it, then a steady state will be reached whereby both solutions have 25 sodium ions and 25 chloride ions. If, however, the porous barrier is selective to which ions are let through, then diffusion alone will not determine the resulting solution. Returning to the previous example, lets now construct a barrier that is only permeable to sodium ions. Since solution B has a lower concentration of both sodium and chloride, it will attract both ions from solution A. However, only sodium will travel through the barrier. This will result in an accumulation of sodium in solution B. Since sodium has a positive charge, this accumulation will make solution B more positive relative to solution A. Positive sodium ions will be less likely to travel to the now more positive B solution. This constitutes the second factor controlling ion flow, namely electric fields. The point at which this electric field completely counteracts the force due to diffusion is called the equilibrium potential. At this point, the net flow of this specific ion (in this case sodium) is zero.

Cell membrane
Each neuron is encased in a cell membrane. This membrane is nearly impermeable to ions.[11] To transfer ions into, and out of the neuron, the membrane provides two structures. Ion pumps use the cell's energy to continuously move ions in and out. They create concentration differences (between the inside and outside of the neuron) by transporting ions against their concentration gradients (from regions of low concentration to regions of high concentration). The ion channels then use this concentration difference to transport ions down their concentration gradients (from regions of high concentration to regions of low concentration). However, unlike the continuous transport by the ion pumps, the transport by the ion channels is non continuous. They only open and close in response to signals from their environment. This transport of ions through the ion channels then changes the voltage of the cell membrane. These changes are what bring about an action potential. As an analogy, ion pumps play the role of the battery that allows a radio circuit (the ion channels) to transmit a signal (action potential).[1]

Membrane potential
The cell membrane acts as a barrier which prevents the inside solution (intracellular fluid) from mixing with the outside solution (extracellular fluid). These two solutions have different concentrations of their ions. Furthermore, this difference in concentrations leads to a difference in charge of the solutions. This creates a situation whereby one solution is more positive than the other. Therefore, positive ions will tend to gravitate towards the negative solution. Likewise, negative ions will tend to gravitate towards the positive solution. To quantify this property, one would like to somehow capture this relative positivity (or negativity). To do this, the outside solution is set as the zero voltage. Then the difference between the inside voltage and the zero voltage is determined. For example, if the outside voltage is 100 mV, and the inside voltage is 30 mV, then the difference is 70 mV. This difference is what is commonly referred to as the membrane potential.
Ion channels
Ion channels are integral membrane proteins through which ions can cross the membrane. Most channels are specific for one ion; whereas that ion passes through relatively quickly, other similar ions pass through very infrequently.[13] For example, although potassium and sodium ions have the same charge and differ only slightly in their radius, potassium channels allow few sodium ions through, and vice versa. The pore through which the ion passes is typically so small that ions must pass through it alone and single-file.[14] Channels are either fully open or fully closed. When the channel is open, ions flow through it by passive transport, i.e., at a rate determined by the membrane potential Vm and concentration difference across the membrane.[15] The action potential is a manifestation of different ion channels opening and closing at different times.[16]

A channel may have several different states (corresponding to different conformations of the protein), but each such state is either open or closed. In general, closed states correspond either to a contraction of the pore—making it impassable to the ion—or to a separate part of the protein stoppering the pore. For example, the voltage-dependent sodium channel undergoes inactivation, in which a portion of the protein swings into the pore, sealing it.[17] This inactivation shuts off the sodium current and plays a critical role in the action potential.
Ion channels can be classified by how they respond to their environment.[18] For example, the ion channels involved in the action potential are voltage-sensitive channels; they open and close in response to the voltage across the membrane. Ligand-gated channels form another important class; these ion channels open and close in response to the binding of a ligand molecule, such as a neurotransmitter. Other ion channels open and close with mechanical forces. Still other ion channels—such as those of sensory neurons—open and close in response to other stimuli, such as light, temperature or pressure.
Ion pumps
The ionic currents of the action potential flow in response to concentration differences of the ions across the cell membrane. These concentration differences are established by ion pumps, which are integral membrane proteins that carry out active transport, i.e., use cellular energy (ATP) to "pump" the ions against their concentration gradient.[19] Such ion pumps take in ions from one side of the membrane (decreasing its concentration there) and release them on the other side (increasing its concentration there). The ion pump most relevant to the action potential is the sodium–potassium pump, which transports three sodium ions out of the cell and two potassium ions in.[20] Consequently, the concentration of potassium ions K+ inside the neuron is roughly 20-fold larger than the outside concentration, whereas the sodium concentration outside is roughly ninefold larger than inside.[21][22] Similarly, other ions have different concentrations inside and outside the neuron, such as calcium, chloride and magnesium.[22]
Ion pumps influence the action potential only by establishing the relative ratio of intracellular and extracellular ion concentrations. The action potential mainly involves the opening and closing of ion channels, not ion pumps. If the ion pumps are turned off by removing their energy source, or by adding an inhibitor such as ouabain, the axon can still fire hundreds of thousands of action potentials before their amplitudes begin to decay significantly.[19] In particular, ion pumps play no significant role in the repolarization of the membrane after an action potential.[7]
Resting potential
As described in the section Ions and the forces driving their motion, the point at which the electric field completely counteracts the force due to diffusion is called the equilibrium potential. The electric field, in turn, is based on the charge of the ion in question. In the case of sodium, the charge is +1. Furthermore, in the case of diffusion, it is based on the difference in concentration between the two solutions. With these two parameters, the equilibrium potential for any ion can be calculated with the Nernst equation[23][24]
The constants in this equation are the charge valence n of the ion (e.g., +1 for K+, +2 for Ca2+ and −1 for Cl−), the temperature T (in Kelvins), the molar gas constant R, and the Faraday F, which is the total negative charge of a mole of electrons. Clearly, even if two different ions have the same charge (ie. K+ and Na+), they can still have drastically different equilibrium potentials, provided their outside and/or inside concentrations differ. Take, for example, the real life equilibrium potentials of potassium and sodium in neurons. The potassium equilibrium potential EK is −75 mV. The sodium equilibrium potential, on the other hand, ENa is +55mV. Since these disagree, there is no voltage at which the net flow of potassium and sodium ions are both zero.[note 1]
However, there is an equilibrium membrane potential Em at which the net flow of all ions across the membrane is zero. This potential is calculated by the Goldman equation[25][26] It is essentially the Nernst equation, in that it is based on the charges of the ions in question, as well as the difference between their inside and outside concentrations. However, it also takes into consideration the relative permeability of the porous membrane to each ion in question.
for the three monovalent ions most important to action potentials: potassium (K+), sodium (Na+), and chloride (Cl−). Being an anion, the chloride terms are treated differently than the cation terms; the inside concentration is in the numerator, and the outside concentration is in the denominator, which is reversed from the cation terms. Pi stands for the permeability of the ion type i. If calcium ions are also considered, the formula for the equilibrium potential becomes more complicated.[27]
The Goldman equation can be used to explain which ions the membrane gives preference to during the resting phase in case of irrelevant pump activity, as in animal cells. Here, the resting membrane is most permeable to potassium, thus one would expect the resting membrane potential Vrest to be close to the potassium equilibrium potential of −75 mV.[28][29] This holds to be true, as Vrest is usually about −70 mV in animal cells.[30]

Anatomy of a neuron
Several types of cells support an action potential, such as plant cells, muscle cells, and the specialized cells of the heart (in which occurs the cardiac action potential). However, the main excitable cell is the neuron, which also has the simplest mechanism for the action potential.
Neurons are electrically excitable cells generally comprised of one or more dendrites, a single soma, a single axon and one or more axon terminals. The dendrite is one of the two types of synapses, the other being the axon terminal buttons. Dendrites form protrusions in response to the axon terminal boutons. These protrusions, or spines, are designed to capture the neurotransmitters released by the presynaptic neuron. They have a high concentration of ligand activated channels. It is, therefore, here where synapses from two neurons communicate with one another. These spines have a thin neck connecting a bulbous protrusion to the main dendrite. This ensures that changes occurring inside the spine are less likely to affect the neighbouring spines. The dendritic spine can, therefore, with rare exception (see LTP), act as an independent unit. The dendrites then connect onto the soma. The soma houses the nucleus, which acts as the regulator for the neuron. Unlike the spines, the surface of the soma is populated by voltage activated ion channels. These channels help transmit the signals generated by the dendrites. Emerging out from the soma is the axon hillock. This region is characterized by having an incredibly high concentration of voltage activated sodium channels. It is generally considered to be the spike initiation zone for action potentials.[31] Multiple signals generated at the spines, and transmitted by the soma all converge here. Immediately after the axon hillock is the axon. This is a thin tubular protrusion traveling away from the soma. The axon is insulated by a myelin sheath. Myelin is composed of Schwann cells that wrap themselves multiple times around the axonal segment. This forms a thick fatty layer that prevents ions from entering or escaping the axon. This insulation both prevents significant signal decay as well as ensuring faster signal speed. This insulation, however, has the restriction that no channels can be present on the surface of the axon. There are, therefore, regularly spaced patches of membrane which have no insulation. These nodes of ranvier can be considered to be 'mini axon hillocks' as their purpose is to boost the signal in order to prevent significant signal decay. At the furthest end, the axon loses its insulation and begins to branch into several axon terminals. These axon terminals then end in the form the second class of synapses, axon terminal buttons. These buttons have voltage activated calcium channels which come into play when signaling other neurons.
| Neuron |
|---|
Initiation
Before considering the propagation of action potentials along axons and their termination at the synaptic knobs, it is helpful to consider the methods by which action potentials can be initiated at the axon hillock. The basic requirement is that the membrane voltage at the hillock be raised above the threshold for firing.[32] There are several ways in which this depolarization can occur.

Neurotransmission
Action potentials are most commonly initiated by excitatory postsynaptic potentials from a presynaptic neuron.[33] Typically, neurotransmitter molecules are released by the presynaptic neuron. These neurotransmitters then bind to receptors on the postsynaptic cell. This binding opens various types of ion channels. This opening has the further effect of changing the local permeability of the cell membrane and thus the membrane potential. If the binding increases the voltage (depolarizes the membrane), the synapse is excitatory. If, however, the binding decreases the voltage (hyperpolarizes the membrane), it is inhibitory. Whether the voltage is decreased or increased, the change propagates passively to nearby regions of the membrane (as described by the cable equation and its refinements). Typically, the voltage stimulus decays exponentially with the distance from the synapse and with time from the binding of the neurotransmitter. Some fraction of an excitatory voltage may reach the axon hillock and may (in rare cases) depolarize the membrane enough to provoke a new action potential. More typically, the excitatory potentials from several synapses must work together at nearly the same time to provoke a new action potential. Their joint efforts can be thwarted, however, by the counter-acting inhibitory postsynaptic potentials.
Neurotransmission can also occur through electrical synapses.[34] Due to the direct connection between the excitable cells in such cases, an action potential can well be transmitted directly from one cell to the next. Rectifying channels ensure that action potentials only move in one direction through an electrical synapse.
"All-or-none" principle
The amplitude of an action potential is independent of the amount of current which produced it. In other words, larger currents do not create larger action potentials. Therefore action potentials are said to be all-or-none, since they either occur fully or they do not occur at all. Instead, the frequency of action potentials is what encodes for the intensity of a stimulus. This is in contrast to receptor potentials, whose amplitudes are dependent on the intensity of a stimulus.[1]
Sensory neurons
In sensory neurons, an external signal such as pressure, temperature, light, or sound is coupled with the opening and closing of ion channels, which in turn alter the ionic permeabilities of the membrane and its voltage.[35] These voltage changes can again be excitatory (depolarizing) or inhibitory (hyperpolarizing) and, in some sensory neurons, their combined effects can depolarize the axon hillock enough to provoke action potentials. Examples in humans include the olfactory receptor neuron and Meissner's corpuscle, which are critical for the sense of smell and touch, respectively. However, not all sensory neurons convert their external signals into action potentials; some do not even have an axon![36] Instead, they may convert the signal into the release of a neurotransmitter, or into continuous graded potentials, either of which may stimulate subsequent neuron(s) into firing an action potential. For illustration, in the human ear, hair cells convert the incoming sound into the opening and closing of mechanically gated ion channels, which may cause neurotransmitter molecules to be released. Similarly, in the human retina, the initial photoreceptor cells and the next two layers of cells (bipolar cells and amacrine cells) do not produce action potentials; only the third layer, the ganglion cells, produce action potentials, which then travel up the optic nerve.
Pacemaker potentials

In the cases of neurotransmission and sensory neurons, action potentials result from an external stimulus. However, some excitable cells require no such stimulus to fire: they spontaneously depolarize their axon hillock and fire action potentials at a regular rate, like an internal clock.[37] The voltage traces of such cells are known as pacemaker potentials.[38] The cardiac pacemaker cells of the sinoatrial node in the heart provide a good example.[39] Although such pacemaker potentials have a natural rhythm, it can be adjusted by external stimuli; for instance, heart rate can be altered by pharmaceuticals as well as signals from the sympathetic and parasympathetic nerves.[40] The external stimuli do not cause the cell's repetitive firing, but merely alter its timing.[38] In some cases, the regulation of frequency can be more complex, leading to patterns of action potentials, such as bursting.
Phases
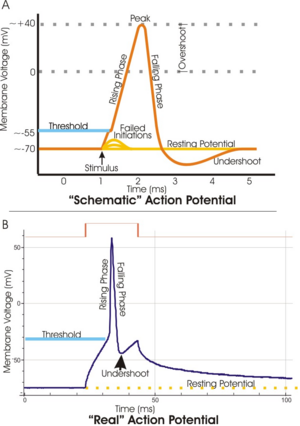
The course of the action potential can be divided into five parts: the rising phase, the peak phase, the falling phase, the undershoot phase, and finally the refractory period. During the rising phase the membrane potential depolarises (becomes more positive). The point at which depolarisation stops is called the peak phase. At this stage, the membrane potential reaches a maximum. Subsequent to this, there is a falling phase. During this stage the membrane potential hyperpolarises (becomes more negative). The undershoot phase is the point during which the membrane potential becomes temporarily more negatively charged than when at rest. Finally, the time during which a subsequent action potential is impossible or difficult to fire is called the refractory period, which may overlap with the other phases.[41]
The course of the action potential is determined by two coupled effects.[42] First, voltage-sensitive ion channels open and close in response to changes in the membrane voltage Vm. This changes the membrane's permeability to those ions.[43] Secondly, according to the Goldman equation, this change in permeability changes in the equilibrium potential Em, and, thus, the membrane voltage Vm.[26] Thus, the membrane potential affects the permeability, which then further affects the membrane potential. This sets up the possibility for positive feedback, which is a key part of the rising phase of the action potential.[3] A complicating factor is that a single ion channel may have multiple internal "gates" that respond to changes in Vm in opposite ways, or at different rates.[44][45] For example, although raising Vm opens most gates in the voltage-sensitive sodium channel, it also closes the channel's "inactivation gate", albeit more slowly.[46] Hence, when Vm is raised suddenly, the sodium channels open initially, but then close due to the slower inactivation.
The voltages and currents of the action potential in all of its phases were modeled accurately by Alan Lloyd Hodgkin and Andrew Huxley in 1952,[45] for which they were awarded the Nobel Prize in Physiology or Medicine in 1963.[47] However, their model considers only two types of voltage-sensitive ion channels, and makes several assumptions about them, e.g., that their internal gates open and close independently of one another. In reality, there are many types of ion channels,[18] and they do not always open and close independently.[48]
Stimulation and rising phase
A typical action potential begins at the axon hillock[49] with a sufficiently strong depolarization, e.g., a stimulus that increases Vm. This depolarization is often caused by the injection of extra sodium cations into the cell; these cations can come from a wide variety of sources, such as chemical synapses, sensory neurons or pacemaker potentials.
The initial membrane permeability to potassium is low, but much higher than that of other ions, making the resting potential close to EK≈ -75 mV.[28] The depolarization opens both the sodium and potassium channels in the membrane, allowing the ions to flow into and out of the axon, respectively. If the depolarization is small (say, increasing Vm from −70 mV to −60 mV), the outward potassium current overwhelms the inward sodium current and the membrane repolarizes back to its normal resting potential around −70 mV.[2] However, if the depolarization is large enough, the inward sodium current increases more than the outward potassium current and a runaway condition (positive feedback) results: the more inward current there is, the more Vm increases, which in turn further increases the inward current.[3] A sufficiently strong depolarization (increase in Vm) causes the voltage-sensitive sodium channels to open; the increasing permeability to sodium drives Vm closer to the sodium equilibrium voltage ENa≈ +55 mV. The increasing voltage in turn causes even more sodium channels to open, which pushes Vm still further towards ENa. This positive feedback continues until the sodium channels are fully open and Vm is close to ENa.[32] The sharp rise in Vm and sodium permeability correspond to the rising phase of the action potential.[32]
The critical threshold voltage for this runaway condition is usually around −45 mV, but it depends on the recent activity of the axon. A membrane that has just fired an action potential cannot fire another one immediately, since the ion channels have not returned to their usual state. The period during which no new action potential can be fired is called the absolute refractory period.[50] At longer times, after some but not all of the ion channels have recovered, the axon can be stimulated to produce another action potential, but only with a much stronger depolarization, e.g., −30 mV. The period during which action potentials are unusually difficult to provoke is called the relative refractory period.[50]
Peak and falling phase
The positive feedback of the rising phase slows and comes to a halt as the sodium ion channels become maximally open. At the peak of the action potential, the sodium permeability is maximized and the membrane voltage Vm is nearly equal to the sodium equilibrium voltage ENa. However, the same raised voltage that opened the sodium channels initially also slowly shuts them off, by closing their pores; the sodium channels become inactivated.[46] This lowers the membrane's permeability to sodium, driving the membrane voltage back down. At the same time, the raised voltage opens voltage-sensitive potassium channels; the increase in the membrane's potassium permeability drives Vm towards EK.[46] Combined, these changes in sodium and potassium permeability cause Vm to drop quickly, repolarizing the membrane and producing the "falling phase" of the action potential.[51][51]
Hyperpolarization ("undershoot")
The raised voltage opened many more potassium channels than usual, and these do not close right away when the membrane returns to its normal resting voltage. The potassium permeability of the membrane is transiently unusually high, driving the membrane voltage Vm even closer to the potassium equilibrium voltage EK. Hence, there is an undershoot, a hyperpolarization in technical language, that persists until the membrane potassium permeability returns to its usual value.[52]
Refractory period
The opening and closing of the sodium and potassium channels during an action potential may leave some of them in a "refractory" state, in which they are unable to open again until they have recovered.[50] In the absolute refractory period, so many ion channels are refractory that no new action potential can be fired. Significant recovery (de-inactivation) requires that the membrane potential remain hyperpolarized for a certain length of time. In the relative refractory period, enough channels have recovered that an action potential can be provoked, but only with a stimulus much stronger than usual. These refractory periods ensure that the action potential travels in only one direction along the axon.[53]
Propagation
The action potential generated at the axon hillock propagates as a wave along the axon.[54] The currents flowing inwards at a point on the axon during an action potential spread out along the axon, and depolarize the adjacent sections of its membrane. If sufficiently strong, this depolarization provokes a similar action potential at the neighboring membrane patches. This basic mechanism was demonstrated by Alan Lloyd Hodgkin in 1937. After crushing or cooling nerve segments and thus blocking the action potentials, he showed that an action potential arriving on one side of the block could provoke another action potential on the other, provided that the blocked segment was sufficiently short.[55]
Once an action potential has occurred at a patch of membrane, the membrane patch needs time to recover before it can fire again. At the molecular level, this absolute refractory period corresponds to the time required for its ion channels to return to their normal open or closed states.[56] Although it limits the frequency of firing,[57] the absolute refractory period ensures that the action potential moves in only one direction along an axon.[53] The currents flowing in due to an action potential spread out in both directions along the axon.[58] However, only the unfired part of the axon can respond with an action potential; the part that has just fired is unresponsive until the action potential is safely out of range and cannot restimulate that part. In the usual orthodromic conduction, the action potential propagates from the axon hillock towards the synaptic knobs (the axonal termini); propagation in the opposite direction—known as antidromic conduction—is very rare.[59] However, if a laboratory axon is stimulated in its middle, both halves of the axon are "fresh", i.e., unfired; then two action potentials will be generated, one traveling towards the axon hillock and the other traveling towards the synaptic knobs.

Myelin and saltatory conduction
The axons of some neurons are ensheathed in myelin, a fatty (ie, lipid-rich) insulating material that increases the speed and energy efficiency of action potential conduction.[60] Axons are myelinated by specialized cells, Schwann cells and oligodendrocytes, that wrap themselves multiple times around segments of axon.[61] The gaps between these segments are known as the nodes of Ranvier.
Myelin prevents ions from entering or leaving the axon along myelinated segments. Myelination is found mainly in vertebrates, but an analogous system has been discovered in a few invertebrates, such as some species of shrimp.[62] As a general rule, myelination increases the conduction velocity of action potentials and makes them more energy-efficient. However, not all neurons in vertebrates are myelinated. Whether saltatory or not, the mean conduction velocity of an action potential ranges from 1 m/s to over 100 m/s, and generally increases with axonal diameter.[63]
Action potentials cannot propagate through the membrane in myelinated segments of the axon. However, the current is carried by the cytoplasm, which is sufficient to depolarize the next 1 or 2 node of Ranvier. Instead, the ionic current from an action potential at one node of Ranvier provokes another action potential at the next node; this apparent "hopping" of the action potential from node to node is known as saltatory conduction. Although the mechanism of saltatory conduction was suggested in 1925 by Ralph Lillie,[64] the first experimental evidence for saltatory conduction came from Ichiji Tasaki[65] and Taiji Takeuchi[66] and from Andrew Huxley and Robert Stämpfli.[67] By contrast, in unmyelinated axons, the action potential provokes another in the membrane immediately adjacent, and moves continuously down the axon like a wave.

Myelin has two important advantages: fast conduction speed and energy efficiency. For axons larger than a minimum diameter (roughly 1 micron), myelination increases the conduction velocity of an action potential, typically tenfold.[70] Conversely, for a given conduction velocity, myelinated fibers are smaller than their unmyelinated counterparts. For example, action potentials move at roughly the same speed (25 m/s) in a myelinated frog axon and an unmyelinated squid giant axon, but the frog axon has a roughly 30-fold smaller diameter and 100-fold smaller cross-sectional area. Also, since the ionic currents are confined to the nodes of Ranvier, far fewer ions "leak" across the membrane, saving metabolic energy. This saving is a significant selective advantage, since the human nervous system uses approximately 20% of the body's metabolic energy.[70]
The length of axons' myelinated segments is important to the success of saltatory conduction. They should be as long as possible to maximize the speed of conduction, but not so long that the arriving signal is too weak to provoke an action potential at the next node of Ranvier. In nature, myelinated segments are generally long enough for the passively propagated signal to travel for at least two nodes while retaining enough amplitude to fire an action potential at the second or third node. Thus, the safety factor of saltatory conduction is high, allowing transmission to bypass nodes in case of injury. However, action potentials may end prematurely in certain places where the safety factor is low, even in unmyelinated neurons; a common example is the branch point of an axon, where it divides into two axons.[71]
Some diseases degrade myelin and impair saltatory conduction, reducing the conduction velocity of action potentials.[72] The most well-known of these is multiple sclerosis, in which the breakdown of myelin impairs coordinated movement.[73]
Cable theory

The flow of currents within an axon can be described quantitatively by cable theory[74] and its elaborations, such as the compartmental model.[75] Cable theory was developed in 1855 by Lord Kelvin to model the transatlantic telegraph cable[76] and was shown to be relevant to neurons by Hodgkin and Rushton in 1946.[77] In simple cable theory, the neuron is treated as an electrically passive, perfectly cylindrical transmission cable, which can be described by a partial differential equation[74]
where V(x, t) is the voltage across the membrane at a time t and a position x along the length of the neuron, and where λ and τ are the characteristic length and time scales on which those voltages decay in response to a stimulus. Referring to the circuit diagram above, these scales can be determined from the resistances and capacitances per unit length[78]
These time- and length-scales can be used to understand the dependence of the conduction velocity on the diameter of the neuron in unmyelinated fibers. For example, the time-scale τ increases with both the membrane resistance rm and capacitance cm. As the capacitance increases, more charge must be transferred to produce a given transmembrane voltage (by the equation Q=CV); as the resistance increases, less charge is transferred per unit time, making the equilibration slower. Similarly, if the internal resistance per unit length ri is lower in one axon than in another (e.g., because the radius of the former is larger), the spatial decay length λ becomes longer and the conduction velocity of an action potential should increase. If the transmembrane resistance rm is increased, that lowers the average "leakage" current across the membrane, likewise causing λ to become longer, increasing the conduction velocity.
Termination
Chemical synapses
Action potentials that reach the synaptic knobs generally cause a neurotransmitter to be released into the synaptic cleft.[79] Neurotransmitters are small molecules that may open ion channels in the postsynaptic cell; most axons have the same neurotransmitter at all of their termini. The arrival of the action potential opens voltage-sensitive calcium channels in the presynaptic membrane; the influx of calcium causes vesicles filled with neurotransmitter to migrate to the cell's surface and release their contents into the synaptic cleft.[80] This complex process is inhibited by the neurotoxins tetanospasmin and botulinum toxin, which are responsible for tetanus and botulism, respectively.[81]

Electrical synapses
Some synapses dispense with the "middleman" of the neurotransmitter, and connect the presynaptic and postsynaptic cells together.[82] When an action potential reaches such a synapse, the ionic currents flowing into the presynaptic cell can cross the barrier of the two cell membranes and enter the postsynaptic cell through pores known as connexins.[83] Thus, the ionic currents of the presynaptic action potential can directly stimulate the postsynaptic cell. Electrical synapses allow for faster transmission because they do not require the slow diffusion of neurotransmitters across the synaptic cleft. Hence, electrical synapses are used whenever fast response and coordination of timing are crucial, as in escape reflexes, the retina of vertebrates, and the heart.
Neuromuscular junctions
A special case of a chemical synapse is the neuromuscular junction, in which the axon of a motor neuron terminates on a muscle fiber.[84] In such cases, the released neurotransmitter is acetylcholine, which binds to the acetylcholine receptor, an integral membrane protein in the membrane (the sarcolemma) of the muscle fiber.[85] However, the acetylcholine does not remain bound; rather, it dissociates and is hydrolyzed by the enzyme, acetylcholinesterase, located in the synapse. This enzyme quickly reduces the stimulus to the muscle, which allows the degree and timing of muscular contraction to be regulated delicately. Some poisons inactivate acetylcholinesterase to prevent this control, such as the nerve agents sarin and tabun,[86] and the insecticides diazinon and malathion.[87]
Other cell types
Cardiac action potentials

The cardiac action potential differs from the neuronal action potential by having an extended plateau, in which the membrane is held at a high voltage for a few hundred milliseconds prior to being repolarized by the potassium current as usual.[88] This plateau is due to the action of slower calcium channels opening and holding the membrane voltage near their equilibrium potential even after the sodium channels have inactivated.
The cardiac action potential plays an important role in coordinating the contraction of the heart.[88] The cardiac cells of the sinoatrial node provide the pacemaker potential that synchronizes the heart. The action potentials of those cells propagate to and through the atrioventricular node (AV node), which is normally the only conduction pathway between the atria and the ventricles. Action potentials from the AV node travel through the bundle of His and thence to the Purkinje fibers.[note 2] Conversely, anomalies in the cardiac action potential—whether due to a congenital mutation or injury—can lead to human pathologies, especially arrhythmias.[88] Several anti-arrhythmia drugs act on the cardiac action potential, such as quinidine, lidocaine, beta blockers, and verapamil.[89]
Muscular action potentials
The action potential in a normal skeletal muscle cell is similar to the action potential in neurons.[90] Action potentials result from the depolarization of the cell membrane (the sarcolemma), which opens voltage-sensitive sodium channels; these become inactivated and the membrane is repolarized through the outward current of potassium ions. The resting potential prior to the action potential is typically −90mV, somewhat more negative than typical neurons. The muscle action potential lasts roughly 2–4 ms, the absolute refractory period is roughly 1–3 ms, and the conduction velocity along the muscle is roughly 5 m/s. The action potential releases calcium ions that free up the tropomyosin and allow the muscle to contract. Muscle action potentials are provoked by the arrival of a pre-synaptic neuronal action potential at the neuromuscular junction, which is a common target for neurotoxins.[86]
Plant action potentials
In principle, plant and fungal cells [91] are also electrically excitable. The fundamental difference to animal action potentials is, that the depolarization in plant cells is not accomplished by an uptake of positive sodium ions, but by release of negative chloride ions [92] [93] [94]. Together with the following release of positive potassium ions, which is common to plant and animal action potentials, the action potential in plants infers, therefore, an osmotic loss of salt (KCl), whereas the animal action potential is osmotically neutral, when equal amounts of entering sodium and leaving potassium cancel each other osmotically. The interaction of electrical and osmotic relations in plant cells [95] indicates an osmotic function of electrical excitability in the common, unicellular ancestors of plants and animals under changing salinity conditions, whereas the present function of rapid signal transmission is seen as a younger accomplishment of metazoan cells in a more stable osmotic environment [96]. It must be assumed that the familiar signalling function of action potentials in some vascular plants (e.g. Mimosa pudica), arose independently from that in metazoan excitable cells.
Taxonomic distribution and evolutionary advantages
Action potentials are found throughout multicellular organisms, including plants, invertebrates such as insects, and vertebrates such as reptiles and mammals.[97] Sponges seem to be the main phylum of multicellular eukaryotes that does not transmit action potentials, although some studies have suggested that these organisms have a form of electrical signaling, too.[98] The resting potential, as well as the size and duration of the action potential, have not varied much with evolution, although the conduction velocity does vary dramatically with axonal diameter and myelination.
| Animal | Cell type | Resting potential (mV) | AP increase (mV) | AP duration (ms) | Conduction speed (m/s) |
|---|---|---|---|---|---|
| Squid (Loligo) | Giant axon | −60 | 120 | 0.75 | 35 |
| Earthworm (Lumbricus) | Median giant fiber | −70 | 100 | 1.0 | 30 |
| Cockroach (Periplaneta) | Giant fiber | −70 | 80–104 | 0.4 | 10 |
| Frog (Rana) | sciatic nerve axon | −60 to −80 | 110–130 | 1.0 | 7–30 |
| Cat (Felis) | Spinal motor neuron | −55 to −80 | 80–110 | 1–1.5 | 30–120 |
Given its conservation throughout evolution, the action potential seems to confer evolutionary advantages. One function of action potentials is rapid, long-range signaling within the organism; the conduction velocity can exceed 110 m/s, which is one-third the speed of sound. No material object could convey a signal that rapidly throughout the body; for comparison, a hormone molecule carried in the bloodstream moves at roughly 8 m/s in large arteries. Part of this function is the tight coordination of mechanical events, such as the contraction of the heart. A second function is the computation associated with its generation. Being an all-or-none signal that does not decay with transmission distance, the action potential has similar advantages to digital electronics. The integration of various dendritic signals at the axon hillock and its thresholding to form a complex train of action potentials is another form of computation, one that has been exploited biologically to form central pattern generators and mimicked in artificial neural networks.
Experimental methods
- See also: Electrophysiology

The study of action potentials has required the development of new experimental methods. The initial work, prior to 1955, focused on three goals: isolating signals from single neurons or axons, developing fast, sensitive electronics, and shrinking electrodes enough that the voltage inside a single cell could be recorded.
The first problem was solved by studying the giant axons found in the neurons of the squid genus Loligo.[100] These axons are so large in diameter (roughly 1 mm, or 100-fold larger than a typical neuron) that they can be seen with the naked eye, making them easy to extract and manipulate.[45][101] However, the Loligo axons are not representative of all excitable cells, and numerous other systems with action potentials have been studied.
The second problem was addressed with the crucial development of the voltage clamp,[102] which permitted experimenters to study the ionic currents underlying an action potential in isolation, and eliminated a key source of electronic noise, the current IC associated with the capacitance C of the membrane.[103] Since the current equals C times the rate of change of the transmembrane voltage Vm, the solution was to design a circuit that kept Vm fixed (zero rate of change) regardless of the currents flowing across the membrane. Thus, the current required to keep Vm at a fixed value is a direct reflection of the current flowing through the membrane. Other electronic advances included the use of Faraday cages and electronics with high input impedance, so that the measurement itself did not affect the voltage being measured.[104]
The third problem, that of obtaining electrodes small enough to record voltages within a single axon without perturbing it, was solved in 1949 with the invention of the glass micropipette electrode,[105] which was quickly adopted by other researchers.[106][107] Refinements of this method are able to produce electrode tips that are as fine as 100 Å (10 nm), which also confers high input impedance.[108] Action potentials may also be recorded with small metal electrodes placed just next to a neuron, with neurochips containing EOSFETs, or optically with dyes that are sensitive to Ca2+ or to voltage.[109]

While glass micropipette electrodes measure the sum of the currents passing through many ion channels, studying the electrical properties of a single ion channel became possible in the 1970s with the development of the patch clamp by Erwin Neher and Bert Sakmann. For this they were awarded the Nobel Prize in Physiology or Medicine in 1991.[110] Patch-clamping verified that ionic channels have discrete states of conductance, such as open, closed and inactivated.
Neurotoxins
Several neurotoxins, both natural and synthetic, are designed to block the action potential. Tetrodotoxin from the pufferfish and saxitoxin from the Gonyaulax (the dinoflagellate genus responsible for "red tides") block action potentials by inhibiting the voltage-sensitive sodium channel;[111] similarly, dendrotoxin from the black mamba snake inhibits the voltage-sensitive potassium channel. Such inhibitors of ion channels serve an important research purpose, by allowing scientists to "turn off" specific channels at will, thus isolating the other channels' contributions; they can also be useful in purifying ion channels by affinity chromatography or in assaying their concentration. However, such inhibitors also make effective neurotoxins, and have been considered for use as chemical weapons. Neurotoxins aimed at the ion channels of insects have been effective insecticides; one example is the synthetic permethrin, which prolongs the activation of the sodium channels involved in action potentials. The ion channels of insects are sufficiently different from their human counterparts that there are few side effects in humans. Many other neurotoxins interfere with the transmission of the action potential's effects at the synapses, especially at the neuromuscular junction.
History
The role of electricity in the nervous systems of animals was first observed in dissected frogs by Luigi Galvani, who studied it from 1791 to 1797.[112] Galvani's results stimulated Alessandro Volta to develop the Voltaic pile—the earliest known electric battery—with which he studied animal electricity (such as electric eels) and the physiological responses to applied direct-current voltages.[113]
Scientists of the 19th century studied the propagation of electrical signals in whole nerves (i.e., bundles of neurons) and demonstrated that nervous tissue was made up of cells, instead of an interconnected network of tubes (a reticulum).[114] Carlo Matteucci followed up Galvani's studies and demonstrated that cell membranes had a voltage across them and could produce direct current. Matteucci's work inspired the German physiologist, Emil du Bois-Reymond, who discovered the action potential in 1848. The conduction velocity of action potentials was first measured in 1850 by du Bois-Reymond's friend, Hermann von Helmholtz. To establish that nervous tissue was made up of discrete cells, the Spanish physician Santiago Ramón y Cajal and his students used a stain developed by Camillo Golgi to reveal the myriad shapes of neurons, which they rendered painstakingly. For their discoveries, Golgi and Ramón y Cajal were awarded the 1906 Nobel Prize in Physiology.[115] Their work resolved a long-standing controversy in the neuroanatomy of the 19th century; Golgi himself had argued for the network model of the nervous system.

The 20th century was a golden era for electrophysiology. In 1902 and again in 1912, Julius Bernstein advanced the hypothesis that the action potential resulted from a change in the permeability of the axonal membrane to ions.[24] Bernstein's hypothesis was confirmed by Ken Cole and Howard Curtis, who showed that membrane conductance increases during an action potential.[116] In 1907, Louis Lapicque suggested that the action potential was generated as a threshold was crossed[117], what would be later shown as a product of the dynamical systems of ionic conductances. In 1949, Alan Hodgkin and Bernard Katz refined Bernstein's hypothesis by considering that the axonal membrane might have different permeabilities to different ions; in particular, they demonstrated the crucial role of the sodium permeability for the action potential.[29] This line of research culminated in the five 1952 papers of Hodgkin, Katz and Andrew Huxley, in which they applied the voltage clamp technique to determine the dependence of the axonal membrane's permeabilities to sodium and potassium ions on voltage and time, from which they were able to reconstruct the action potential quantitatively.[45] Hodgkin and Huxley correlated the properties of their mathematical model with discrete ion channels that could exist in several different states, including "open", "closed", and "inactivated". Their hypotheses were confirmed in the mid-1970s and 1980s by Erwin Neher and Bert Sakmann, who developed the technique of patch clamping to examine the conductance states of individual ion channels.[118] In the 21st century, researchers are beginning to understand the structural basis for these conductance states and for the selectivity of channels for their species of ion,[119] through the atomic-resolution crystal structures,[14] fluorescence distance measurements[120] and cryo-electron microscopy studies.[121]
Julius Bernstein was also the first to introduce the Nernst equation for resting potential across the membrane; this was generalized by David E. Goldman to the eponymous Goldman equation in 1943.[26] The sodium–potassium pump was identified in 1957[122] and its properties gradually elucidated,[19][20][123] culminating in the determination of its atomic-resolution structure by X-ray crystallography.[124] The crystal structures of related ionic pumps have also been solved, giving a broader view of how these molecular machines work.[125]
Quantitative models

Mathematical and computational models are essential for understanding the action potential, and offer predictions that may be tested against experimental data, providing a stringent test of a theory. The most important and accurate of these models is the Hodgkin–Huxley model, which describes the action potential by a coupled set of four ordinary differential equations (ODEs).[45] Although the Hodgkin–Huxley model may be a simplification of a realistic nervous membrane, its complexity has inspired several even-more-simplified models,[126] such as the Morris–Lecar model[127] and the FitzHugh–Nagumo model,[128] both of which have only two coupled ODEs. The properties of the Hodgkin–Huxley and FitzHugh–Nagumo models and their relatives, such as the Bonhoeffer–van der Pol model,[129] have been well-studied within mathematics,[130] computation[131] and electronics.[132] More modern research has focused on larger and more integrated systems; by joining action-potential models with models of other parts of the nervous system (such as dendrites and synapses), researches can study neural computation[133] and simple reflexes, such as escape reflexes and others controlled by central pattern generators.[134]
See also
- Bursting
- Signals (biology)
- Central pattern generator
Notes
- ↑ Membrane potentials are defined relative to the exterior of the cell; thus, a potential of −70 mV implies that the interior of the cell is negative relative to the exterior.
- ↑ Note that these Purkinje fibers are muscle fibers and not related to the Purkinje cells, which are neurons found in the cerebellum.
References
- ↑ 1.0 1.1 1.2 Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed.. Sinauer Associates. pp. 7, 27-28. ISBN 978-0-87893-697-7.
- ↑ 2.0 2.1 Bullock, Orkand, and Grinnell, pp. 150–151; Junge, pp. 89–90; Schmidt-Nielsen, p. 484.
- ↑ 3.0 3.1 3.2 Purves et al., pp. 48–49; Bullock, Orkand, and Grinnell, pp. 141, 150–151; Schmidt-Nielsen, p. 483; Junge, p. 89; Stevens, p. 127
- ↑ Schmidt-Nielsen, p. 484.
- ↑ Bullock, Orkand, and Grinnell, p. 209.
- ↑ Johnston and Wu, p. 9.
- ↑ 7.0 7.1 Bullock, Orkand, and Grinnell, pp. 140–41.
- ↑ Bullock, Orkand, and Grinnell, pp. 153–54.
- ↑ Mummert H, Gradmann D (1991). "Action potentials in Acetabularia: measurement and simulation of voltage-gated fluxes". Journal of Membrane Biology 124: 265–73. PMID 1664861.
- ↑ Schmidt-Nielsen, p. 483.
- ↑ Lieb WR, Stein WD (1986). "Chapter 2. Simple Diffusion across the Membrane Barrier". Transport and Diffusion across Cell Membranes. San Diego: Academic Press. pp. 69–112. ISBN 0-12-664661-9.
- ↑ CRC Handbook of Chemistry and Physics, 83rd edition, ISBN 0-8493-0483-0, pp. 12–14 to 12–16.
- ↑ Eisenman G (1961). "On the elementary atomic origin of equilibrium ionic specificity". in A Kleinzeller, A Kotyk, eds.. Symposium on Membrane Transport and Metabolism. New York: Academic Press. pp. 163–79.
* Eisenman G (1965). "Some elementary factors involved in specific ion permeation". Proc. 23rd Int. Congr. Physiol. Sci., Tokyo. Amsterdam: Excerta Med. Found.. pp. 489–506.
* Diamond JM, Wright EM (1969). "Biological membranes: the physical basis of ion and nonekectrolyte selectivity". Annual Review of Physiology 31: 581–646. doi:. - ↑ 14.0 14.1 Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, et al. (1998). "The structure of the potassium channel, molecular basis of K+ conduction and selectivity". Science 280: 69–77. doi:. PMID 9525859.
* Zhou Y, Morias-Cabrak JH, Kaufman A, MacKinnon R (2001). "Chemistry of ion coordination and hydration revealed by a K+-Fab complex at 2.0 A resolution". Nature 414: 43–48. doi:.
* Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R (2003). "X-ray structure of a voltage-dependent K+ channel". Nature 423: 33–41. doi:. - ↑ Junge, pp. 33–37.
- ↑ Bullock, Orkand, and Grinnell, p. 132.
- ↑ Cai SQ, Li W, Sesti F (2007). "Multiple modes of a-type potassium current regulation". Curr. Pharm. Des. 13 (31): 3178–84. doi:. PMID 18045167.
- ↑ 18.0 18.1 Goldin AL (2007). "Neuronal Channels and Receptors". in Waxman SG. Molecular Neurology. Burlington, MA: Elsevier Academic Press. pp. 43–58. ISBN 978-0-12-369509-3.
- ↑ 19.0 19.1 19.2 Hodgkin AL, Keynes RD (1955). "Active transport of cations in giant axons from Sepia and Loligo". J. Physiol. 128: 28–60.
- ↑ 20.0 20.1 Caldwell PC, Hodgkin AL, Keynes RD, Shaw TI (1960). "The effects of injecting energy-rich phosphate compounds on the active transport of ions in the giant axons of Loligo". J. Physiol. 152: 561–90.
- ↑ Steinbach HB, Spiegelman S (1943). "The sodium and potassium balance in squid nerve axoplasm". J. Cell. Comp. Physiol. 22: 187–96. doi:.
- ↑ 22.0 22.1 Hodgkin AL (1951). "The ionic basis of electrical activity in nerve and muscle". Biol. Rev. 26: 339–409. doi:.
- ↑ Purves et al., pp. 28–32; Bullock, Orkand, and Grinnell, pp. 133–134; Schmidt-Nielsen, pp. 478–480, 596–597; Junge, pp. 33–35
- ↑ 24.0 24.1 Bernstein J (1902). "Untersuchungen zur Thermodynamik der bioelektrischen Ströme". Pflüger's Arch. Ges. Physiol. 92: 521–562.
* Bernstein J (1912). Elektrobiologie. Braunschweig: Vieweg und Sohn. - ↑ Purves et al., pp. 32–33; Bullock, Orkand, and Grinnell, pp. 138–140; Schmidt-Nielsen, pp. 480; Junge, pp. 35–37
- ↑ 26.0 26.1 26.2 Goldman DE (1943). "Potential, impedance and rectification in membranes". J. Gen. Physiol. 27: 37–60. doi:.
- ↑ Spangler SG (1972). "Expansion of the constant field equation to include both divalent and monovalent ions". Ala J Med Sci 9: 218–23. PMID 5045041.
- ↑ 28.0 28.1 Purves et al., p. 34; Bullock, Orkand, and Grinnell, p. 134; Schmidt-Nielsen, pp. 478–480.
- ↑ 29.0 29.1 Hodgkin AL, Katz B (1949). "The effect of sodium ions on the electrical activity of the giant axon of the squid". J. Physiology 108: 37–77.
- ↑ Purves et al., pp. 33–36; Bullock, Orkand, and Grinnell, p. 131.
- ↑ Bullock, Orkand, and Grinnell, p. 11.
- ↑ 32.0 32.1 32.2 Purves et al., pp. 49–50; Bullock, Orkand, and Grinnell, pp. 140–141, 150–151; Schmidt-Nielsen, pp. 480–481, 483–484; Junge, pp. 89–90.
- ↑ Bullock, Orkand, and Grinnell, pp. 177–240; Schmidt-Nielsen, pp. 490–499; Stevens, pp. 47–68.
- ↑ Bullock, Orkand, and Grinnell, pp. 178–180; Schmidt-Nielsen, pp. 490–491.
- ↑ Schmidt-Nielsen, pp. 535–580; Bullock, Orkand, and Grinnell, pp. 49–56, 76–93, 247–255; Stevens, 69–79
- ↑ Bullock, Orkand, and Grinnell, pp. 53, 122–124.
- ↑ Junge, pp. 115–132
- ↑ 38.0 38.1 Bullock, Orkand, and Grinnell, pp. 152–153.
- ↑ Noble D (1960). "Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations". Nature 188: 495–497. doi:.
- ↑ Bullock, Orkand, and Grinnell, pp. 444–445.
- ↑ Purves et al., p. 38.
- ↑ Stevens, pp. 127–128.
- ↑ Purves et al., pp. 61–65.
- ↑ Purves et al., pp. 64–74; Bullock, Orkand, and Grinnell, pp. 149–150; Junge, pp. 84–85; Stevens, pp. 152–158.
- ↑ 45.0 45.1 45.2 45.3 45.4 Hodgkin AL, Huxley AF, Katz B (1952). "Measurements of current-voltage relations in the membrane of the giant axon of Loligo". Journal of Physiology 116: 424–448. PMID 14946713.
* Hodgkin AL, Huxley AF (1952). "Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo". Journal of Physiology 116: 449–472. PMID 14946713.
* Hodgkin AL, Huxley AF (1952). "The components of membrane conductance in the giant axon of Loligo". J Physiol 116: 473–496. PMID 14946714.
* Hodgkin AL, Huxley AF (1952). "The dual effect of membrane potential on sodium conductance in the giant axon of Loligo". J Physiol 116: 497–506. PMID 14946715.
* Hodgkin AL, Huxley AF (1952). "A quantitative description of membrane current and its application to conduction and excitation in nerve". J Physiol 117: 500–544. PMID 12991237. - ↑ 46.0 46.1 46.2 Purves et al., pp. 47, 65; Bullock, Orkand, and Grinnell, pp. 147–148; Stevens, p. 128.
- ↑ The Nobel Prize in Physiology or Medicine 1963.
- ↑ Naundorf B, Wolf F, Volgushev M (April 2006). "Unique features of action potential initiation in cortical neurons" (Letter). Nature 440: 1060–1063. doi:. http://www.nature.com/nature/journal/v440/n7087/abs/nature04610.html. Retrieved on 2008-03-27.
- ↑ Stevens, p. 49.
- ↑ 50.0 50.1 50.2 Purves et al., p. 49; Bullock, Orkand, Grinell, p. 151; Stevens, pp. 19–20; Junge, pp. 4–5.
- ↑ 51.0 51.1 Purves et al., p. 49; Bullock, Orkand, and Grinnell, pp. 147–149, 152; Schmidt-Nielsen, pp. 483–484; Stevens, pp. 126–127.
- ↑ Purves et al., p. 37; Bullock, Orkand, and Grinnell, p. 152.
- ↑ 53.0 53.1 Purves et al., p. 56.
- ↑ Bullock, Orkland, and Grinnell, pp. 160–64.
- ↑ Hodgkin AL (1937). "Evidence for electrical transmission in nerve, Part I". Journal of Physiology 90: 183–210.
* Hodgkin AL (1937). "Evidence for electrical transmission in nerve, Part II". Journal of Physiology 90: 211–32. - ↑ Stevens, pp. 19–20.
- ↑ Stevens, pp. 21–23.
- ↑ Bullock, Orkand, and Grinnell, pp. 161–164.
- ↑ Bullock, Orkand, and Grinnell, p. 509.
- ↑ Zalc B (2006). "The acquisition of myelin: a success story". Novartis Found. Symp. 276: 15–21; discussion 21–5, 54–7, 275–81. doi:. PMID 16805421.
- ↑ Simons M, Trotter J (October 2007). "Wrapping it up: the cell biology of myelination". Curr. Opin. Neurobiol. 17 (5): 533–40. doi:. PMID 17923405.
- ↑ Xu K, Terakawa S (August 1999). "Fenestration nodes and the wide submyelinic space form the basis for the unusually fast impulse conduction of shrimp myelinated axons". J. Exp. Biol. 202 (Pt 15): 1979–89. PMID 10395528. http://jeb.biologists.org/cgi/pmidlookup?view=long&pmid=10395528.
- ↑ 63.0 63.1 Hursh JB (1939). "Conduction velocity and diameter of nerve fibers". American Journal of Physiology 127: 131–39.
- ↑ Lillie RS (1925). "Factors affecting transmission and recovery in passive iron nerve model". J. Gen. Physiol. 7: 473–507. doi:. See also Keynes and Aidley, p. 78.
- ↑ Tasaki I (1939). "Electro-saltatory transmission of nerve impulse and effect of narcosis upon nerve fiber". Amer. J. Physiol. 127: 211–27.
- ↑ Tasaki I, Takeuchi T (1941). "Der am Ranvierschen Knoten entstehende Aktionsstrom und seine Bedeutung für die Erregungsleitung". Pflüger's Arch. Ges. Physiol. 244: 696–711. doi:.
* Tasaki I, Takeuchi T (1942). "Weitere Studien über den Aktionsstrom der markhaltigen Nervenfaser und über die elektrosaltatorische Übertragung des nervenimpulses". Pflüger's Arch. Ges. Physiol. 245: 764–82. doi:.
* Tasaki I (1959). J Field, HW Magoun, VC Hall. ed.. Handbook of Physiology: Neurophysiology ((sect. 1, vol. 1) ed.). Washington, D.C.: American Physiological Society. pp. pp. 75–121. - ↑ Huxley A, Stämpfli R (1949). "Evidence for saltatory conduction in peripheral myelinated nerve-fibers". Journal of Physiology 108: 315–39.
* Huxley A, Stämpfli R (1949). "Direct determination of membrane resting potential and action potential in single myelinated nerve fibers". Journal of Physiology 112: 476–95. - ↑ Schmidt-Nielsen, Figure 12.13.
- ↑ Rushton WAH (1951). "A theory of the effects of fibre size in the medullated nerve". Journal of Physiology 115: 101–22.
- ↑ 70.0 70.1 Hartline DK, Colman DR (2007). "Rapid conduction and the evolution of giant axons and myelinated fibers". Curr. Biol. 17 (1): R29–R35. doi:. PMID 17208176.
- ↑ Bullock, Orkland, and Grinnell, p. 163.
- ↑ Miller RH, Mi S (2007). "Dissecting demyelination". Nat. Neurosci. 10 (11): 1351–54. doi:. PMID 17965654.
- ↑ Waxman SG (2007). "Multiple Sclerosis as a Neurodegenerative Disease". in Waxman SG. Molecular Neurology. Burlington, MA: Elsevier Academic Press. pp. 333–46. ISBN 978-0-12-369509-3.
- ↑ 74.0 74.1 Rall W (1989). "Cable Theory for Dendritic Neurons". in C. Koch and I. Segev. Methods in Neuronal Modeling: From Synapses to Networks. Cambridge MA: Bradford Books, MIT Press. pp. pp. 9–62. ISBN 0-262-11133-0.
- ↑ Segev I, Fleshman JW, Burke RE (1989). "Compartmental Models of Complex Neurons". in C. Koch and I. Segev. Methods in Neuronal Modeling: From Synapses to Networks. Cambridge MA: Bradford Books, MIT Press. pp. pp. 63–96. ISBN 0-262-11133-0.
- ↑ Kelvin WT (1855). "On the theory of the electric telegraph". Proceedings of the Royal Society 7: 382–99.
- ↑ Hodgkin AL, Rushton WAH (1946). "The electrical constants of a crustacean nerve fibre". Proceedings of the Royal Society B 133: 444–79.
- ↑ Purves et al., pp. 52–53.
- ↑ Süudhof TC (2008). "Neurotransmitter release". Handb Exp Pharmacol 184 (184): 1–21. doi:. PMID 18064409.
- ↑ Rusakov DA (August 2006). "Ca2+-dependent mechanisms of presynaptic control at central synapses". Neuroscientist 12 (4): 317–26. doi:. PMID 16840708.
- ↑ Humeau Y, Doussau F, Grant NJ, Poulain B (May 2000). "How botulinum and tetanus neurotoxins block neurotransmitter release". Biochimie 82 (5): 427–46. doi:. PMID 10865130.
- ↑ Zoidl G, Dermietzel R (2002). "On the search for the electrical synapse: a glimpse at the future". Cell Tissue Res. 310 (2): 137–42. doi:. PMID 12397368.
- ↑ Brink PR, Cronin K, Ramanan SV (1996). "Gap junctions in excitable cells". J. Bioenerg. Biomembr. 28 (4): 351–8. doi:. PMID 8844332.
- ↑ Hirsch NP (July 2007). "Neuromuscular junction in health and disease". Br J Anaesth 99 (1): 132–8. doi:. PMID 17573397. http://bja.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=17573397.
- ↑ Hughes BW, Kusner LL, Kaminski HJ (April 2006). "Molecular architecture of the neuromuscular junction". Muscle Nerve 33 (4): 445–61. doi:. PMID 16228970.
- ↑ 86.0 86.1 Newmark J (2007). "Nerve agents". Neurologist 13 (1): 20–32. doi:. PMID 17215724.
- ↑ Costa LG (2006). "Current issues in organophosphate toxicology". Clin. Chim. Acta 366 (1-2): 1–13. doi:. PMID 16337171.
- ↑ 88.0 88.1 88.2 Kléber AG, Rudy Y (April 2004). "Basic mechanisms of cardiac impulse propagation and associated arrhythmias". Physiol. Rev. 84 (2): 431–88. doi:. PMID 15044680. http://physrev.physiology.org/cgi/pmidlookup?view=long&pmid=15044680.
- ↑ Tamargo J, Caballero R, Delpón E (January 2004). "Pharmacological approaches in the treatment of atrial fibrillation". Curr. Med. Chem. 11 (1): 13–28. doi:. PMID 14754423.
- ↑ Ganong W (1991). Review of Medical Physiology (15th edition ed.). Norwalk CT: Appleton and Lange. pp. pp. 59–60. ISBN 0-8385-8418-7.
- ↑ Slayman CL, Long WS, Gradmann D (1976). "Action potentials in Neurospora crassa , a mycelial fungus". Biochimica et biophysica acta 426: 737-744. PMID 130926.
- ↑ Mummert H, Gradmann D (1991). "Action potentials in Acetabularia: measurement and simulation of voltage-gated fluxes". Journal of Membrane Biology 124: 265-273. PMID 1664861.
- ↑ Gradmann D (2001). "Models for oscillations in plants". Austr. J. Plant Physiol. 28: 577- 590.
- ↑ Beilby MJ (2007). "Action potentials in charophytes". Int. Rev. Cytol. 257: 43-82. doi:. PMID 17280895.
- ↑ Gradmann D, Hoffstadt J (1998). "Electrocoupling of ion transporters in plants: Interaction with internal ion concentrations". Journal of Membrane Biology 166: 51-59. PMID 9784585.
- ↑ Gradmann D, Mummert H (1980). "Plant action potentials". in Spanswick RM, Lucas WJ, Dainty J. Plant Membrane Transport: Current Conceptual Issues. Amsterdam: Elsevier Biomedical Press. pp. 333-344. ISBN 0444801928.
- ↑ Fromm J, Lautner S (2007). "Electrical signals and their physiological significance in plants". Plant Cell Environ. 30 (3): 249–257. doi:. PMID 17263772.
- ↑ Leys SP, Mackie GO, Meech RW (1999). "Impulse conduction in a sponge". J. Exp. Biol. 202 (Pt 9): 1139–50. PMID 10101111. http://jeb.biologists.org/cgi/pmidlookup?view=long&pmid=10101111.
- ↑ Bullock TH, Horridge GA (1965). Structure and Function in the Nervous Systems of Invertebrates. San Francisco: W. H. Freeman.
- ↑ Keynes RD (1989). "The role of giant axons in studies of the nerve impulse". BioEssays 10: 90–93. doi:. PMID 2541698.
- ↑ Meunier C, Segev I (2002). "Playing the devil's advocate: is the Hodgkin-Huxley model useful?". Trends Neurosci. 25 (11): 558–63. doi:. PMID 12392930.
- ↑ Cole KS (1949). "Dynamic electrical characteristics of the squid axon membrane". Arch. Sci. Physiol. 3: 253–8.
- ↑ Junge, pp. 63–82.
- ↑ Kettenmann H, Grantyn R (1992). Practical Electrophysiological Methods. New York: Wiley. ISBN 978-0471562009.
- ↑ Ling G, Gerard RW (1949). "The normal membrane potential of frog sartorius fibers". J. Cell. Comp. Physiol. 34: 383–396. doi:. PMID 15410483.
- ↑ Nastuk WL, Hodgkin AL (1950). "The electrical activity of single muscle fibers". J. Cell. Comp. Physiol. 35: 39–73. doi:.
- ↑ Brock LG, Coombs JS, Eccles JC (1952). "The recording of potentials from motoneurones with an intracellular electrode". J. Physiol. (London) 117: 431–460.
- ↑ Snell FM (1969). "Some Electrical Properties of Fine-Tipped Pipette Microelectrodes". in M. Lavallée, OF Schanne, NC Hébert. Glass Microelectrodes. New York: John Wiley and Sons. LCCN 68-9252.
- ↑ Ross WN, Salzberg BM, Cohen LB, Davila HV (1974). "A large change in dye absorption during the action potential". Biophysical Journal 14: 983–986.
* Grynkiewicz G, Poenie M, Tsien RY (1985). "A new generation of Ca2+ indicators with greatly improved fluorescence properties". J. Biol. Chem. 260: 3440–3450. - ↑ The Nobel Prize in Physiology or Medicine 1991.
- ↑ Nakamura Y, Nakajima S, Grundfest H (1965). "The ffect of tetrodotoxin on electrogenic components of squid giant axons". J. Gen. Physiol. 48: 985–996. doi:.
* Ritchie JM, Rogart RB (1977). "The binding of saxitoxin and tetrodotoxin to excitable tissue". Rev. Physiol. Biochem. Pharmacol. 79: 1–50. doi:.
* Keynes RD, Ritchie JM (1984). "On the binding of labelled saxitoxin to the squid giant axon". Proc. R. Soc. Lond. 239: 393–434. - ↑ Piccolino M (1997). "Luigi Galvani and animal electricity: two centuries after the foundation of electrophysiology". Trends in Neuroscience 20: 443–448. doi:.
- ↑ Piccolino M (2000). "The bicentennial of the Voltaic battery (1800–2000): the artificial electric organ". Trends in Neuroscience 23: 147–151. doi:.
- ↑ Brazier MAB (1961). A History of the Electrical Activity of the Brain. London: Pitman.
* McHenry LC (1969). Garrison's History of Neurology. Springfield, IL: Charles C. Thomas.
* Swazey J, Worden FG (1975). Paths of Discovery in the Neurosciences. Cambridge, MA: The MIT Press. - ↑ The Nobel Prize in Physiology or Medicine 1906.
- ↑ Cole KS, Curtis HJ (1939). "Electrical impedance of the squid giant axon during activity". J. Gen. Physiol. 22: 649–670. doi:.
- ↑ "Recherches quantitatives sur l’excitationelectrique des nerfs traitee comme une polarisation". J. Physiol. Pathol. Gen 9: 620– 635. 1907.
- ↑ Neher E, Sakmann B (1976). "Single-channel currents recorded from membrane of denervated frog muscle fibres". Nature 260: 779–802.
* Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981). "Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches". Pflugers Arch. 391: 85–100. doi:.
* Neher E, Sakmann B (1992). "The patch clamp technique". Scientific American 266: 44–51. - ↑ Yellen G (2002). "The voltage-gated potassium channels and their relatives". Nature 419: 35–42. doi:.
- ↑ Cha A, Snyder GE, Selvin PR, Bezanilla F (1999). "Atomic-scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy". Nature 402: 809–813. doi:.
* Glauner KS, Mannuzzu LM, Gandhi CS, Isacoff E (1999). "Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel". Nature 402: 813–817. doi:.
* Bezanilla F (2000). "The voltage sensor in voltage-dependent ion channels". Physiol. Rev. 80: 555–592. - ↑ Catterall WA (2001). "A 3D view of sodium channels". Nature 409: 988–999. doi:.
* Sato C, Ueno Y, Asai K, Takahashi K, Sato M, Engel A, et al. (2001). "The voltage-sensitive sodium channel is a bell-shaped molecule with several cavities". Nature 409: 1047–1051. doi:. - ↑ Skou J (1957). "The influence of some cations on an adenosine triphosphatase from peripheral nerves". Biochim Biophys Acta 23 (2): 394–401. doi:. PMID 13412736.
* The Nobel Prize in Chemistry 1997. Nobelprize.org. Retrieved on 2007-04-21. - ↑ Caldwell PC, Keynes RD (1957). "The utilization of phosphate bond energy for sodium extrusion from giant axons". J. Physiol. (London) 137: 12–13P.
- ↑ Morth JP, Pedersen PB, Toustrup-Jensen MS, Soerensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P (2007). "Crystal structure of the sodium–potassium pump". Nature 450: 1043–1049. doi:.
- ↑ Lee AG, East JM (2001). "What the structure of a calcium pump tells us about its mechanism". Biochemical Journal 356: 665–683. doi:. PMID 11389676.
- ↑ Hoppensteadt FC (1986). An introduction to the mathematics of neurons. Cambridge: Cambridge University Press. ISBN 0-521-31574-3.
* FitzHugh R (1960). "Thresholds and plateaus in the Hodgkin-Huxley nerve equations". J. Gen. Physiol. 43: 867–896. doi:. PMID 13823315.
* Kepler TB, Abbott LF (1992). "Reduction of conductance-based neuron models". Biological Cybernetics 66: 381–387. doi:. - ↑ Morris C, Lecar H (1981). "Voltage oscillations in the barnacle giant muscle fiber". Biophysical Journal 35: 193–213.
- ↑ FitzHugh R (1961). "Impulses and physiological states in theoretical models of nerve membrane". Biophysical Journal 1: 445–466.
* Nagumo J, Arimoto S, Yoshizawa S (1962). "An active pulse transmission line simulating nerve axon". Proceedings of the IRE 50: 2061–2070. doi:. - ↑ Bonhoeffer KF (1948). "Activation of Passive Iron as a Model for the Excitation of Nerve". J. Gen. Physiol. 32: 69–91. doi:.
* Bonhoeffer KF (1953). "Modelle der Nervenerregung". Naturwissenschaften 40: 301–311. doi:.
* van der Pol B (1926). "On relaxation-oscillations". Philosophical Magazine 2: 978–992.
* van der Pol B, van der Mark J (1928). "The heartbeat considered as a relaxation oscillation, and an electrical model of the heart". Philosophical Magazine 6: 763–775.
* van der Pol B, van der Mark J (1929). "The heartbeat considered as a relaxation oscillation, and an electrical model of the heart". Arch. Neerl. Physiol. 14: 418–443. - ↑ Sato S, Fukai H, Nomura T, Doi S (2005). "Bifurcation Analysis of the Hodgkin-Huxley Equations". in Reeke GN, Poznanski RR, Lindsay KA, Rosenberg JR, Sporns O. Modeling in the Neurosciences: From Biological Systems to Neuromimetic Robotics (2nd edition ed.). Boca Raton: CRC Press. pp. pp. 459–478. ISBN 978-0415328685.
* Evans JW (1972). "Nerve axon equations. I. Linear approximations". Indiana U. Math. Journal 21: 877–885. doi:.
* Evans JW, Feroe J (1977). "Local stability theory of the nerve impulse". Math. Biosci. 37: 23–50. doi:.
* FitzHugh R (1969). "Mathematical models of axcitation and propagation in nerve". in HP Schwann. Biological Engineering. New York: McGraw-Hill. pp. pp. 1–85.
* Guckenheimer J, Holmes P (1986). Nonlinear Oscillations, Dynamical Systems and Bifurcations of Vector Fields (2nd printing, revised and corrected ed.). New York: Springer Verlag. pp. pp. 12–16. ISBN 0-387-90819-6. - ↑ Nelson ME, Rinzel J (1994). "The Hodgkin-Huxley Model". in Bower J, Beeman D. The Book of GENESIS: Exploring Realistic Neural Models with the GEneral NEural SImulation System. New York: Springer Verlag. pp. pp. 29–49.
* Rinzel J, Ermentrout GB (1989). "Analysis of Neural Excitability and Oscillations". in C. Koch, I Segev. Methods in Neuronal Modeling: From Synapses to Networks. Cambridge, MA: Bradford Book, The MIT Press. pp. pp. 135–169. ISBN 0-262-11133-0. - ↑ Keener JP (1983). "Analogue circuitry for the van der Pol and FitzHugh-Nagumo equations". IEEE Trans. On Systems, Man and Cybernetics 13: 1010–1014.
- ↑ McCulloch WS (1988). Embodiments of Mind. Cambridge MA: The MIT Press. pp. pp. 19–39, 46–66, 72–141. ISBN 0-262-63114-8.
* JA Anderson, E Rosenfeld, ed.. Neurocomputing:Foundations of Research. Cambridge, MA: The MIT Press. pp. 15–41. ISBN 0-262-01097-6. - ↑ Getting PA (1989). "Reconstruction of Small Neural Networks". in C Koch and I Segev. Methods in Neuronal Modeling: From Synapses to Networks. Cambridge, MA: Bradford Book, The MIT Press. pp. pp. 171–194. ISBN 0-262-11133-0.
* Hooper, Scott L. "Central Pattern Generators." Embryonic ELS (1999) http://www.els.net/elsonline/figpage/I0000206.html (2 of 2) [2/6/2001 11:42:28 AM] Online: Accessed 27 November 2007 [1]
Bibliography
- Aidley DJ, Stanfield PR (1996). Ion Channels: Molecules in Action. Cambridge: Cambridge University Press. ISBN 978-0521498821.
- Bear MF, Connors BW, Paradiso MA (2001). Neuroscience: Exploring the Brain. Baltimore: Lippincott. ISBN 0781739446.
- Bullock TH, Orkand R, Grinnell A (1977). Introduction to Nervous Systems. New York: W. H. Freeman. ISBN 0-7167-0030-1.
- Clay JR (May 2005). "Axonal excitability revisited". Prog Biophys Mol Biol 88 (1): 59–90. doi:. PMID 15561301.
- Deutsch S, Micheli-Tzanakou E (1987). Neuroelectric Systems. New York: New York University Press. ISBN 0-8147-1782-9.
- Hille B (2001). Ion Channels of Excitable Membranes (3rd edition ed.). Sunderland, MA: Sinauer Associates. ISBN 978-0878933211.
- Hoppensteadt FC (1986). An Introduction to the Mathematics of Neurons. Cambridge: Cambridge University Press. ISBN 0-521-31574-3.
- Johnston D, Wu SM-S (1995). Foundations of Cellular Neurophysiology. Cambridge, MA: Bradford Book, The MIT Press. ISBN 0-262-10053-3.
- Junge D (1981). Nerve and Muscle Excitation (2nd ed. ed.). Sunderland MA: Sinauer Associates. ISBN 0-87893-410-3.
- Kandel ER, Schwartz JH, Jessell TM (2000). Principles of Neural Science (4th ed. ed.). New York: McGraw-Hill. ISBN 0-8385-7701-6.
- Keynes RD, Aidley DJ (1991). Nerve and Muscle (2nd ed. ed.). Cambridge: Cambridge University Press. ISBN 0-521-41042-8.
- Miller C (1987). "How ion channel proteins work". in LK Kaczmarek, IB Levitan. Neuromodulation: The Biochemical Control of Neuronal Excitability. New York: Oxford University Press. pp. pp. 39–63. ISBN 978-0195040975.
- Nelson DL, Cox MM (2008). Lehninger Principles of Biochemistry (5th ed. ed.). New York: W. H. Freeman. ISBN 978-0-7167-7108-1.
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A-S, McNamara JO, Williams SM (2001). "Release of Transmitters from Synaptic Vesicles". Neuroscience (2nd ed. ed.). Sunderland, MA: Sinauer Associates. ISBN 0878937250.
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A-S, McNamara JO, White LE (2008). Neuroscience (4th ed. ed.). Sunderland, MA: Sinauer Associates. ISBN 978-0-87893-697-7.
- Schmidt-Nielsen K (1997). Animal Physiology: Adaptation and Environment (5th ed. ed.). Cambridge: Cambridge University Press. ISBN 978-0521570985.
- Stevens CF (1966). Neurophysiology: A Primer. New York: John Wiley and Sons.LCCN 66-15872.
External links
- Animations
- Ionic flow in action potentials at Blackwell Publishing
- Action potential propagation in myelinated and unmyelinated axons at Blackwell Publishing
- Generation of AP in cardiac cells and generation of AP in neuron cells
- Resting membrane potential from Life: The Science of Biology, by WK Purves, D Sadava, GH Orians, and HC Heller, 8th edition, New York: WH Freeman, ISBN 978-0716776710.
- Ionic motion and the Goldman voltage for arbitrary ionic concentrations at University of Arizona
- A cartoon illustrating the action potential
- Lecture notes and other materials
- The Action Potential John Kinnamon, University of Denver
- Resting and Action Membrane Potentials Teaching Resources Center, UC Davis. Animated tutorials
- Open-source software to simulate neuronal and cardiac action potentials at SourceForge.net
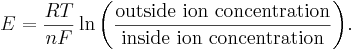
![E_{m} = \frac{RT}{F} \ln{ \left( \frac{ P_{\mathrm{K}}[\mathrm{K}^{+}]_\mathrm{out} + P_{\mathrm{Na}}[\mathrm{Na}^{+}]_\mathrm{out} + P_{\mathrm{Cl}}[\mathrm{Cl}^{-}]_\mathrm{in}}{ P_{\mathrm{K}}[\mathrm{K}^{+}]_\mathrm{in} + P_{\mathrm{Na}}[\mathrm{Na}^{+}]_\mathrm{in} + P_{\mathrm{Cl}}[\mathrm{Cl}^{-}]_\mathrm{out}} \right) }](/2009-wikipedia_en_wp1-0.7_2009-05/I/5939d2ef41c086b20b25a948677b9305.png)