Acid rain
| Air pollution |
| Acid rain • Air Quality Index • Atmospheric dispersion modeling • Chlorofluorocarbon • Global dimming • Global distillation• Global warming • Indoor air quality • Ozone depletion • Particulate • Smog |
| Water pollution |
| Eutrophication • Hypoxia • Marine pollution • Marine debris • Ocean acidification • Oil spill • Ship pollution • Surface runoff • Thermal pollution • Wastewater • Waterborne diseases • Water quality • Water stagnation • |
| Soil contamination |
| Bioremediation • Electrical resistance heating • Herbicide • Pesticide • Soil Guideline Values (SGVs) |
| Radioactive contamination |
| Actinides in the environment • Environmental radioactivity • Fission product • Nuclear fallout • Plutonium in the environment • Radiation poisoning • Radium in the environment • Uranium in the environment |
| Other types of pollution |
| Invasive species • Light pollution • Noise pollution • Radio spectrum pollution • Visual pollution |
| Inter-government treaties |
| Montreal Protocol • Kyoto Protocol • CLRTAP • OSPAR • Stockholm Convention |
| Major organizations |
| DEFRA • EPA • Global Atmosphere Watch • EEA • Greenpeace • American Lung Association |
| Related topics |
| Environmental Science • Natural environment • Acid Rain Program |
Acid rain is rain or any other form of precipitation that is unusually acidic. It has harmful effects on plants, aquatic animals, and infastructure. Acid rain is mostly caused by human emissions of sulfur and nitrogen compounds which react in the atmosphere to produce acids. In recent years, many governments have introduced laws to reduce these emissions.
Contents |
Definition
"Acid rain" is a popular term referring to the deposition of wet (rain, snow, sleet, fog and cloudwater, dew) and dry (acidifying particles and gases) acidic components. A more accurate term is “acid deposition”. Distilled water, which contains no carbon dioxide, has a neutral pH of 7. Liquids with a pH less than 7 are acidic, and those with a pH greater than 7 are basic. “Clean” or unpolluted rain has a slightly acidic pH of about 5.2, because carbon dioxide and water in the air react together to form carbonic acid, a weak acid (pH 5.6 in distilled water), but unpolluted rain also contains other chemicals.[1]
Carbonic acid then can ionize in water forming low concentrations of hydronium ions:
The extra acidity in rain comes from the reaction of primary air pollutants, primarily sulfur oxides and nitrogen oxides, with water in the air to form strong acids (like sulfuric and nitric acid). The main sources of these pollutants are industrial power-generating plants and vehicles.
History
Since the Industrial Revolution, emissions of sulphur dioxide and nitrogen oxides to the atmosphere have increased.[2] [3] In 1852, Robert Angus Smith was the first to show the relationship between acid rain and atmospheric pollution in Manchester, England.[4] Though acidic rain was discovered in 1852, it wasn't until the late 1960s that scientists began widely observing and studying the phenomenon. The term "acid rain" was generated in 1972.[5] Canadian Harold Harvey was among the first to research a "dead" lake. Public awareness of acid rain in the U.S increased in the 1970s after the New York Times promulgated reports from the Hubbard Brook Experimental Forest in New Hampshire of the myriad deleterious environmental effects demonstrated to result from it.[6][7]
Occasional pH readings in rain and fog water of well below 2.4 (the acidity of vinegar) have been reported in industrialized areas.[2] Industrial acid rain is a substantial problem in Europe, China,[8][9] Russia and areas down-wind from them. These areas all burn sulfur-containing coal to generate heat and electricity.[10] The problem of acid rain not only has increased with population and industrial growth, but has become more widespread. The use of tall smokestacks to reduce local pollution has contributed to the spread of acid rain by releasing gases into regional atmospheric circulation.[11][12] Often deposition occurs a considerable distance downwind of the emissions, with mountainous regions tending to receive the greatest deposition (simply because of their higher rainfall). An example of this effect is the low pH of rain (compared to the local emissions) which falls in Scandinavia.[13]
Emissions of chemicals leading to acidification
The most important gas which leads to acidification is sulfur dioxide. Emissions of nitrogen oxides which are oxidized to form nitric acid are of increasing importance due to stricter controls on emissions of sulfur containing compounds. 70 Tg(S) per year in the form of SO2 comes from fossil fuel combustion and industry, 2.8 Tg(S) from wildfires and 7-8 Tg(S) per year from volcanoes.[14]
Natural phenomena
The principal natural phenomena that contribute acid-producing gases to the atmosphere are emissions from volcanoes and those from biological processes that occur on the land, in wetlands, and in the oceans. The major biological source of sulfur containing compounds is dimethyl sulfide.
Acidic deposits have been detected in glacial ice thousands of years old in remote parts of the globe.[15]
Human activity
The principal cause of acid rain is sulfur and nitrogen compounds from human sources, such as electricity generation, factories, and motor vehicles. Coal power plants are one of the most polluting. The gases can be carried hundreds of kilometres in the atmosphere before they are converted to acids and deposited. In the past, factories had short funnels to let out smoke, but this caused many problems locally; thus, factories now have taller smoke funnels. However, dispersal from these taller stacks causes pollutants to be carried farther, causing widespread ecological damage.
Chemical processes
Gas phase chemistry
In the gas phase sulfur dioxide is oxidized by reaction with the hydroxyl radical via an intermolecular reaction:
- SO2 + OH· → HOSO2·
which is followed by:
- HOSO2· + O2 → HO2· + SO3
In the presence of water, sulfur trioxide (SO3) is converted rapidly to sulfuric acid:
- SO3(g) + H2O(l) → H2SO4(l)
Nitric acid is formed by the reaction of OH with nitrogen dioxide:
- NO2 + OH· → HNO3
For more information see Seinfeld and Pandis (1998).[4]
Chemistry in cloud droplets
When clouds are present the loss rate of SO2 is faster than can be explained by gas phase chemistry alone. This is due to reactions in the liquid water droplets
- Hydrolysis
Sulfur dioxide dissolves in water and then, like carbon dioxide, hydrolyses in a series of equilibrium reactions:
- SO2 (g)+ H2O
 SO2·H2O
SO2·H2O - SO2·H2O
 H++HSO3-
H++HSO3- - HSO3-
 H++SO32-
H++SO32-
- Oxidation
There are a large number of aqueous reactions that oxidize sulfur from S(IV) to S(VI), leading to the formation of sulfuric acid. The most important oxidation reactions are with ozone, hydrogen peroxide and oxygen (reactions with oxygen are catalyzed by iron and manganese in the cloud droplets).
For more information see Seinfeld and Pandis (1998).[4]
Acid deposition
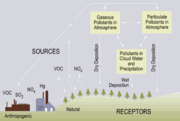
Wet deposition
Wet deposition of acids occurs when any form of precipitation (rain, snow, etc.) removes acids from the atmosphere and delivers it to the Earth's surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosols are both of importance for wet deposition.
Dry deposition
Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition.[16] This occurs when particles and gases stick to the ground, plants or other surfaces.
Adverse effects

Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic lifeforms as well as causing damage to buildings and having impacts on human health.
Surface waters and aquatic animals
Both the lower pH and higher aluminum concentrations in surface water that occur as a result of acid rain can cause damage to fish and other aquatic animals. At pHs lower than 5 most fish eggs will not hatch and lower pHs can kill adult fish. As lakes and rivers become more acidic biodiversity is reduced. Acid rain has eliminated insect life and some fish species, including the brook trout in some lakes, streams, and creeks in geographically sensitive areas, such as the Adirondack Mountains of the United States.[17] However, the extent to which acid rain contributes directly or indirectly via runoff from the catchment to lake and river acidity (i.e., depending on characteristics of the surrounding watershed) is variable. The United States Environmental Protection Agency's (EPA) website states: "Of the lakes and streams surveyed, acid rain caused acidity in 75 percent of the acidic lakes and about 50 percent of the acidic streams".[17]
Soils
Soil biology and chemistry can be seriously damaged by acid rain. Some microbes are unable to tolerate changes to low pHs and are killed. [18] The enzymes of these microbes are denatured (changed in shape so they no longer function) by the acid. The hydronium ions of acid rain also mobilize toxins, e.g. aluminium, and leach away essential nutrients and minerals.[19]
Soil chemistry can be dramatically changed when base cations, such as calcium and magnesium, are leached by acid rain thereby affecting sensitive species, such as sugar maple (Acer saccharum).[20][21]
Forests and other vegetation
Adverse effects may be indirectly related to acid rain, like the acid's effects on soil (see above) or high concentration of gaseous precursors to acid rain. High altitude forests are especially vulnerable as they are often surrounded by clouds and fog which are more acidic than rain.
Other plants can also be damaged by acid rain but the effect on food crops is minimized by the application of lime and fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. When calcium is leached from the needles of red spruce, these trees become less cold tolerant and exhibit winter injury and even death[22][23].
Human health
Scientists have suggested direct links to human health.[24]. Fine particles, a large fraction of which are formed from the same gases as acid rain (sulfur dioxide and nitrogen dioxide), have been shown to cause illness and premature deaths such as cancer and other diseases[25] For more information on the health effects of aerosols see particulate health effects.
Other adverse effects

Acid rain can also cause damage to certain building materials and historical monuments. This results when the sulfuric acid in the rain chemically reacts with the calcium compounds in the stones (limestone, sandstone, marble and granite) to create gypsum, which then flakes off.
- CaCO3 (s) + H2SO4 (aq)
 CaSO4 (aq) + CO2 (g) + H2O (l)
CaSO4 (aq) + CO2 (g) + H2O (l)
This result is also commonly seen on old gravestones where the acid rain can cause the inscription to become completely illegible. Acid rain also causes an increased rate of oxidation for iron.[26] Visibility is also reduced by sulfate and nitrate aerosols and particles in the atmosphere.[27]
Affected areas
Particularly badly affected places around the globe include most of Europe (particularly Scandinavia with many lakes with acidic water containing no life and many trees dead) many parts of the United States (states like New York are very badly affected) and South Western Canada. Other affected areas include the South Eastern coast of China and Taiwan.
Potential problem areas in the future
Places like much of South Asia (Indonesia, Malaysia and Thailand), Western South Africa (the country), Southern India and Sri Lanka and even West Africa (countries like Ghana, Togo and Nigeria) could all be prone to acidic rainfall in the future.
Prevention methods
Technical solutions
In the United States, many coal-burning power plants use Flue gas desulfurization (FGD) to remove sulfur-containing gases from their stack gases. An example of FGD is the wet scrubber which is commonly used in the U.S. and many other countries. A wet scrubber is basically a reaction tower equipped with a fan that extracts hot smoke stack gases from a power plant into the tower. Lime or limestone in slurry form is also injected into the tower to mix with the stack gases and combine with the sulfur dioxide present. The calcium carbonate of the limestone produces pH-neutral calcium sulfate that is physically removed from the scrubber. That is, the scrubber turns sulfur pollution into industrial sulfates.
In some areas the sulfates are sold to chemical companies as gypsum when the purity of calcium sulfate is high. In others, they are placed in landfill. However, the effects of acid rain can last for generations, as the effects of pH level change can stimulate the continued leaching of undesirable chemicals into otherwise pristine water sources, killing off vulnerable insect and fish species and blocking efforts to restore native life.
Automobile emissions control reduces emissions of nitrogen oxides from motor vehicles.
International treaties
A number of international treaties on the long range transport of atmospheric pollutants have been agreed e.g. Sulphur Emissions Reduction Protocol under the Convention on Long-Range Transboundary Air Pollution.
Emissions trading
In this regulatory scheme, every current polluting facility is given or may purchase on an open market an emissions allowance for each unit of a designated pollutant it emits. Operators can then install pollution control equipment, and sell portions of their emissions allowances they no longer need for their own operations, thereby recovering some of the capital cost of their investment in such equipment. The intention is to give operators economic incentives to install pollution controls.
The first emissions trading market was established in the United States by enactment of the Clean Air Act Amendments of 1990. The overall goal of the Acid Rain Program established by the Act[28] is to achieve significant environmental and public health benefits through reductions in emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx), the primary causes of acid rain. To achieve this goal at the lowest cost to society, the program employs both regulatory and market based approaches for controlling air pollution.
See also
- List of environmental issues
- Lists of environmental topics
- Basic Rain
References
- ↑ Likens, G. E., W. C. Keene, J. M. Miller and J. N. Galloway. 1987. Chemistry of precipitation from a remote, terrestrial site in Australia. J. Geophys. Res. 92(D11):13,299-13,314.
- ↑ 2.0 2.1 New Science Directorate Bio Mass Burning Redirect
- ↑ Weathers, K. C. and G. E. Likens. 2006. Acid rain. pp. 1549–1561. In: W. N. Rom (ed.). Environmental and Occupational Medicine. Lippincott-Raven Publ., Philadelphia. Fourth Edition.
- ↑ 4.0 4.1 4.2 Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0-471-17816-0
- ↑ Likens, G. E., F. H. Bormann and N. M. Johnson. 1972. Acid rain. Environment 14(2):33-40.
- ↑ Likens, G. E. and F. H. Bormann. 1974. Acid rain: a serious regional environmental problem. Science 184(4142):1176–1179.
- ↑ Search the HBES Publications
- ↑ Galloway, J. N., Zhao Dianwu, Xiong Jiling and G. E. Likens. 1987. Acid rain: a comparison of China, United States and a remote area. Science 236:1559–1562.
- ↑ CHINA: Industrialization pollutes its country side with Acid Rain
- ↑ [1]
- ↑ Likens, G. E., R. F. Wright, J. N. Galloway and T. J. Butler. 1979. Acid rain. Sci. Amer. 241(4):43-51.
- ↑ Likens, G. E. 1984. Acid rain: the smokestack is the “smoking gun.” Garden 8(4):12-18.
- ↑ http://www.emep.int/publ/common_publications.html
- ↑ Berresheim, H.; Wine, P.H. and Davies D.D., (1995). Sulfur in the Atmosphere. In Composition, Chemistry and Climate of the Atmophere, ed. H.B. Singh. Van Nostran Rheingold ISBN
- ↑ Likens, G. E., R. F. Wright, J. N. Galloway and T. J. Butler. 1979. Acid rain. Sci. Amer. 241(4):43-51.
- ↑ UK National Air Quality Archive: Air Pollution Glossary
- ↑ 17.0 17.1 US EPA: Effects of Acid Rain - Surface Waters and own Aquatic Animals
- ↑ Rodhe, H., et al. The global distribution of acidifying wet deposition. Environmental Science and TEchnology. vlo. 36, no. 20 (October) p. 4382-8
- ↑ US EPA: Effects of Acid Rain - Forests
- ↑ Likens, G. E., C. T. Driscoll, D. C. Buso, M. J. Mitchell, G. M. Lovett, S. W. Bailey, T. G. Siccama, W. A. Reiners and C. Alewell. 2002. The biogeochemistry of sulfur at Hubbard Brook. Biogeochemistry 60(3):235-316.
- ↑ Likens, G. E., C. T. Driscoll and D. C. Buso. 1996. Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272:244-246.
- ↑ DeHayes, D.H., Schaberg, P.G., and G.R. Strimbeck. 2001. Red Spruce Hardiness and Freezing Injury Susceptibility. In: F. Bigras, ed. Conifer Cold Hardiness. Kluwer Academic Publishers, the Netherlands.
- ↑ Lazarus, B. E., P. G. Schaberg, G. Hawley and D. H. DeHayes. 2006. Landscape-scale spatial patterns of winter injury to red spruce foliage in a year of heavy region-wide injury. Can. J. For. Res. 36:142-152.
- ↑ Introduction | Acid Rain | New England | US EPA
- ↑ US EPA: Effects of acid rain - human health.
- ↑ US EPA: Effects of Acid Rain - Materials
- ↑ US EPA: Effects of Acid Rain - Visibility
- ↑ Clean Air Act Amendments of 1990, 42 U.S. Code 7651
Further reading
- John McCormick, Acid Earth: The Global Threat of Acid Pollution (London: Earthscan, 1989) ISBN 185383033X
- Likens, G. E., R. F. Wright, J. N. Galloway and T. J. Butler. 1979. Acid rain. Sci. Amer. 241(4):43-51.
- Weathers, K. C. and G. E. Likens. 2006. Acid rain. pp. 1549–1561. In: W. N. Rom (ed.). Environmental and Occupational Medicine. Lippincott-Raven Publ., Philadelphia. Fourth Edition.
External links
- National Acid Precipitation Assessment Program Report - a 98-page report to Congress
- Acid rain for schools
- Acid rain for schools - Hubbard Brook
- U.S. Environmental Protection Agency - New England Acid Rain Program (superficial)
- Acid Rain (more depth than ref. above)
- U.S. Geological Survey - What is acid rain?
- Acid rain analysis - freeware for simulation and evaluation of titration curves and pH calculations
- CBC Digital Archives – Acid Rain: Pollution and Politics
- Larssen, Thorjørn et al. “Acid Rain in China”. Environmental Science and Technology, 40:2, 2006, pp 418-425.
- Acid Rain: A Continuing National Tragedy - a report from The Adirondack Council on acid rain in the Adirondack region
- Assortment of Summaries on Acid Rain
- Acid rain linked to decline of the wood thrush, a songbird of the Eastern Forest